One-Pot Four Component Reaction for the Synthesis of Formazans in an Environmentally Benign Procedure Mediated by KHSO4 ()
1. Introduction
Formazans were first reported by Von Pechmann [1] and by Bamberger [2] . They are intense coloured dyes characterized by their prominent π-π* transitions and these transitions are sensitive to the nature of the substituent present in the phenyl rings, nature of the organic solvents in solution and the acidity and basicity of the medium [3] -[5] . Extensive studies have been carried out relating to their structure evaluation, photochromatic transitions, tautomer formation, redox potential and organometallic chemistry [6] . Formazans also find extensive use in analytical chemistry [7] [8] and their photochemical and thermochemical properties have been investigated [9] . Formazans are reported to exhibit a wide spectrum of biological activities and notable among them are antiviral [10] [11] , antimicrobial [12] [13] , anti inflammatory, analgesic [14] antifungal [15] properties. Synthetic methods for their preparation have been reviewed by Nineham earlier [16] . Their synthesis is based on two general procedures. The first is the reaction of aryldiazonium salts with phenylhydrazones of aldehydes in basic medium and the second type is based on coupling of aryldiazonium ions with active methylene groups followed by Japp Klingermann rearrangement [17] . However, these methods gave multiple products. Further to obtain the formazans in satisfactory yield strict control of pH of the medium is necessary. Newer methods of synthesis are reported and notable among them are the use of solid-liquid phase transfer catalysts [18] -[21] , liquid-liquid systems and crown ethers [22] , methods reported by Tezcan et al. [23] -[26] and a green method using solid Lewis acid namely nano BF3-SiO2 [27] . The commonly used method of synthesis involves three steps namely preparation of the aldehydehydrazones and the diazonium ions separately before reacting both in a suitable medium to give the formazan. This multiplicity of steps decreases the yield. Further diazotization is the key step and it requires the use of strong mineral acids and low temperature. The reaction conditions are stringent and require fine control of temperature as well as pH. High cost of starting materials, use of corrosive mineral acids and volatile organic solvents makes the procedures unacceptable from the standpoint of green and sustainable chemistry. Further in some instances autocatalytic side reactions tend to lower the yield due to multiple products formation [27] . In some reported synthetic methods, alkali was used to accelerate the reaction which again makes the procedure unacceptable. While the only solvent free protocol reported by Bamoniri et al. [28] appears to be an improvement over other procedures; however, the use of NaOH and BF3-etherate in this method, prompts us to look for alternative green procedures which concomitantly result in high yields of the target product. It has already been reported that diazocoupling reactions could be conveniently carried in a one pot solvent free procedure using KHSO4 with microwave as the promoter in a green synthetic protocol [29] . The reported results encouraged us to extend this protocol and examined its applicability to the synthesis of formazans in a one pot solvent free four component reaction using this acidic salt.
2. Results and Discussion
We first examined the possibility by carrying out a one pot solvent free synthesis of formazans by mixing 4-nitroaniline, benzaldehyde and phenylhydrazine, NaNO2, KHSO4 and 2 mL of deionized water. The mixture was ground to a fine homogeneous product. Gradual development of an intense red colour during grinding lead us to conclude that the reaction could be performed by grinding only however on examining the product in prepared silicagel plates using petroleum ether (60 - 80): ethylacetate (9:1) as the eluent revealed only partial conversion. In order to drive the reaction to completion, the homogeneous mixture obtained after grinding, was subjected to microwave irradiation at 350 Watt power. Higher wattage of microwave was avoided as a precautionary measure against possible explosion because of the proven explosive proclivities of diazonium ions which are formed as the transient intermediate. Complete conversion to the formazan was observed after one minute of exposure. Encouraged by the results, we extended the reaction to the synthesis of a variety of formazans by using several combinations of aromatic aldehydes, aromatic amines and phenylhydrazone and in all cases yields were high and the products could be recovered in a simple work up procedure. The four component solvent free synthesis of formazans is shown in Scheme 1 and their physical characteristics summarized in Table 1.
A comparative study of the present methods to a few other procedures reported in literature is summarized in Table 2. Comparison reveals the superiority and better environmental acceptability of the one pot microwave mediated solvent free synthesis of formazans using cheap and easily available KHSO4.
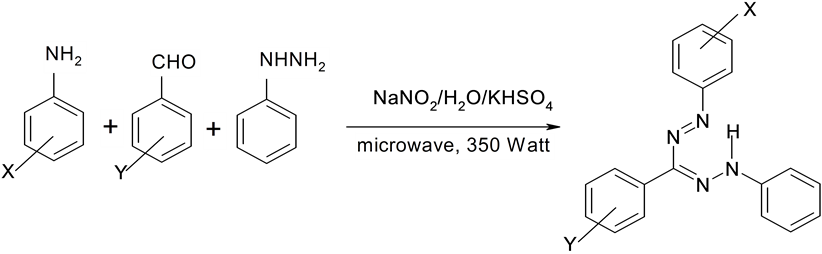
Scheme 1. Microwave mediated one pot solvent free synthesis of Formazans using KHSO4.
![]()
Table 1. Physical characteristics of formazans obtained by a four component solid phase reaction using KHSO4.
![]()
Table 2. Comparison of different methods of the synthesis of formazans.
3. Conclusion
In all the previously reported synthesis of formazan, either the aldehyde phenylhydrazones or the diazonium ions or both were prepared separately and brought into reaction in a suitable medium. In this work, the possibility of a one pot procedure in the absence of any organic solvent or added coupling agents was explored. The use of moist KHSO4 resulted in a one pot environmentally benign synthesis of formazans in high yield and in a very short reaction time. Further, delicate control of temperature as well as pH was not necessary making the procedure suitable for industrial application for large scale environmentally benign production of formazans dyes.
4. Experimental Section
4.1. General
Chemicals were purchased from Loba chieme (India) and purified by procedures reported in literature [32] . Formazans obtained were confirmed by comparing their melting points with those reported in literature. Melting points were recorded in open capillaries and are uncorrected. Products were purified by repeated column chromatography. UV-vis spectra were recorded in UV-1800 Shimadzu UV spectrophotometer, IR spectra were recorded in KBr pellets in a Perkin Elmer FT-IR 1600 spectrophotometer and 1H and 13C NMR were recorded in Bruker Bio Spin 300MHz spectrometer using CDCl3 as solvent and TMS as internal standard. Mass spectra of new compounds were recorded in Micromass QTOF ESI-MS instrument (model HAB273) and Microwave irradiation of reaction mixture was performed in reactor procured from Catalyst™ (India).
4.2. General Procedure for Synthesis of Formazan in the Solid Phase
A mixture of aromatic aldehyde (1 mmol), phenylhydrazine (1 mmol), aromatic amine (1 mmol) and NaNO2 (1.2 mmol), 10 mol% of KHSO4 and 2 mL of deionized water was ground to a homogeneous mixture. The bright coloured mixture was exposed to microwave irradiation (350 Watt) for 60 sec. After completion of the reaction, the solid mass obtained was washed with water till free of KHSO4. The remaining solid was dissolved in ethylacetate, dried using anhydrous Na2SO4 and product obtained by reduced pressure evaporation of the solvent. Finally the products were recrystallized from 95% (v/v) EtOH. All formazans synthesized gave characteristics π-π* absorption in the visible region at 350 to 450 nm which on oxidation with dil HNO3 at room temperature or with 5% KMnO4 solution, shifted the absorption maxima to around 300 nm or below indicating their conversion to tetrazolium salts [5] . This was a confirmative test for the formation of formazans as the product. Table 3 summarizes the shift in the UV absorption maxima of selected formazans after oxidation leading to the formation of tetrazolium salts. The UV spectra of selected formazans before and after oxidation shown in Figure 1 and Figure 2, a and b respectively.
IR spectra of formazans gave the characteristic absorption at 1410 - 1420 cm−1 which is characteristic of the azo group. Several aromatic aldehydes with both electron withdrawing as well as electron releasing groups were examined and the reaction time as well as the yields in all cases were observed to be independent of the electronic effects of the groups present in the benzene rings of both the aromatic amine as well as the aldehyde. We have thus demonstrated that formazans could be successfully synthesized in a one pot procedure starting from arylaldehydes, arylamines, phenylhydrazine and sodium nitrite promoted by KHSO4 without having to synthesize the hydrazones or the diazonium ions separately. The reaction could be carried out without acids or bases consequently control of pH and reaction temperature was not necessary. The yields were high vis-a-vis the wet reaction where several by products are usually obtained resulting in low yield. Further, the present synthetic protocol appears to be more economical and less harmful from environmental point of view. It is expected that this solvent free protocol will be ideally suited for large scale industrial production of a variety of formazan dyes at reduced cost. The physical and spectral data of some selected formazans are listed as the following.
![]()
Table 3. Shift in the π-π* electronic transitions before and after oxidation of formazans.
![]()
Figure 1. UV max of formazan (entry 10) before and after oxidation in ethanol solution.
![]()
Figure 2. UV max of formazan (entry 17) before and after oxidation in ethanol solution.
1-(4-nitrophenyl)-3,5-diphenylformazan (Entry 9): dark red, UV(EtOH): λmax 413 (K-band), IR (KBr): ν cm−1 3094 (Ar-C-H), 2970 (N-H), 1651 (-C=C-), 1556 (-NO2), 1415 (-N=N-), 1303 (-NO2) 1H-NMR (300 MHz, CDCl3): δH ppm 6.979 (1H, d J = 7.6 Hz, NH), 7.140 - 8.496 (m, 14H, Ar-H)), 13C NMR (75 MHz, CDCl3): δ ppm. 114.72, 118.28, 120.43, 121.9, 123.48, 125.18, 127.99, 128.57, 132.37, 133.58, 134.31, 145.19, 150.01 HRMS (ESI): 344.612 calc. 243. HRMS (ESI): 346.128 (M+) calc. 345.
1-(3-nitrophenyl)-3,5-diphenylformazan (Entry 10): dark red, UV(EtOH): λmax 341 nm (K-band), IR (KBr): ν cm−1 3000 (N-H str), 2369 (C-H str), 1653 (-C=C-), 1548 (-NO2), 1417 (-N=N-), 1302 (-NO2), 1H-NMR (300 MHz, CDCl3): δH ppm 7.227 (1H, s, N-H), 7.253 - 8.025 (14H, m, Ar-H), 13CNMR (75 MHz CDCl3): δ ppm 112.30, 112.30, 113.24, 114.49, 116.98, 119.00, 126.52, 128.73, 129.19, 129.93, 138.44, 139.52, 143.58, 145.19, HRMS (ESI): 346.128 (M+) calc. 345.35.
1-(2-nitrophenyl)-3,5-diphenylformazan (Entry 11): red, UV(EtOH): λmax 331.91 nm (K-band), R (KBr): ν cm−1 3346 (Ar-C-H), 3086 (N-H), 1648 (-C=C-), 1555 (-NO2), 1419 (-N=N-), 1302 (-NO2), 1H-NMR (300 MHz, CDCl3): δH ppm 6.883 (1H, t, J = 7.8, J = 7.5, NH), 7.759 - 8.203 (14H, m, Ar-H), 13C NMR (75 MHz, CDCl3): δ ppm. 116.47, 118.49, 121.01, 126.19, 127.25, 129.02, 130.04, 134.49, 136.34, 137.02, 142.16, 143.9, 147.84, 149.34, HRMS (ESI): 344.612 obs, 345 calc.
1-(2,4-dichlorophenyl)-3,5-diphenylformazan (Entry 12): dark red, UV (EtOH): λmax 374.96 nm (K band), IR (KBr): ν cm−1 3276 (C-H str), 3000 (N-H str), 1642 (-C=C-), 1556 (C-Cl), 1414 (-N=N-), 1H-NMR (300 MHz, CDCl3): δH ppm 7.193 (1H, s, NH) 7.612 - 7.639 (13H, m, Ar-H), 13C NMR (CDCl3, 75 MHz): 77.41 115.43, 118.95, 123.20, 123.62, 128.41, 128.98, 129.47, 129.70, 130.33, 130.56, 130.78, 131.07, 133.49, 145.12, HRMS (ESI): 369.0617 (M+, Obs) 369.25 (Calc.).
1-(4-acetoxyphenyl)-3,5-diphenylformazan (Entry 13): dark red, UV (EtOH): λmax 371 (K-band), IR (KBr): ν cm−1 2987 (N-H str), 2351 (aro C-H), 1658 (-CO-), 1417 (-N=N-), 1H-NMR (300 MHz, CDCl3): δH ppm 6.619 (1H, s, N-H), 7.648 - 7.681 (14H, m, Ar-H), 2.524 (3H,-COCH3) 13C NMR (CDCl3, 75 MHz): 26.03, 112.59, 113.60, 119.87, 126.05, 127.50, 128.27, 128.50, 129.20, 130.75, 135.25, 137.18, 144.60, 151.19, 196.73 HRMS (ESI): 342.32 (M+, Obs) 342.39 (Calc).
1-(4-methylphenyl)-3,5-diphenylformazan (Entry 14): dark red, UV(EtOH): λmax 346.23 (K-band), IR (KBr): ν cm−1 2994 (N-H), 1644 (-C=C-), 1556 (-C=N-), 1412 (-N=N-), 1H-NMR (300 MHz, CDCl3) δH ppm 2.258 (3H, s), 6.625 (1H, d, J = 8.4, NH), 7.004 - 8.24 (14H, m, Ar-H), 13C NMR (75 MHz, CDCl3) δ ppm 146.81, 143.55, 141.76, 133.73, 129.92, 129.72, 129.38, 129.22, 127.88, 126.12, 124.03, 123.94, 123.56, 121.1, 115.31, 113, 20.42. HRMS (ESI): 344.612 (Obs) 243 (Calc.).
1-(4-chlorophenyl)-3-(4-methylphenyl)-5-phenyl formazan (Entry 15): orange red, UV(EtOH): λmax 355.12 nm (K-band), IR (KBr): ν cm−1 3001(N-H str), 2364 (C-H str), 1650 (-C=C-), 1414 (-N=N-), 1H-NMR (300 MHz, CDCl3): δH ppm 1.798 (3H, s, CH3), 6.622 (1H, s, N-H) 6.650 - 7.627 (13H, m, Ar-H), 13CNMR (75 MHz, CDCl3): δ ppm 20.13, 117.29, 118.59, 122.27, 124.65, 126.80, 127.60, 28.81, 130.05, 132.11, 137.25, 138.97, 144.62, 151.12, HRMS (ESI): 349.872 (Obs) 348.5 (Calc).
1-(4-chlorophenyl)-3-(2-methoxyphenyl)-5-phenylformazanc (Entry 16): dark red, UV (EtOH): λmax 353.18 nm (K-band), IR (KBr): ν cm−1 2923 (N-H), 1651 (-C-O-), 1556 (-C=N), 1419 (-N=N-), 1H-NMR (300 MHz, CDCl3): δH ppm 3.933 (3H, d, J = 13.4), 6.559 (1H, s, N-H), 6.587 - 8.125 (13H, m, Ar-H) 13CNMR (75 MHz, CDCl3): δ ppm 50.02, 112.72, 20.28, 126.43, 126.80, 128.28, 128.99, 129.25, 129.57, 132.37, 132.58, 133.31, 144.19, 147.58, 148.79, HRMS (ESI): 349.321 (M+) obs, 348.5 calc.
1-(4-methoxyphenyl)-3-(2-chlorophenyl-5-phenylformazan (Entry 17): dark red, UV (EtOH): λmax 353.18 (K-band), IR (KBr): ν cm−1 2985 (N-H), 2363 (-C-H), 1651 (C-O) 1599 (C=N str), 1415 (-N=N-) 1H-NMR (300 MHz, CDCl3): δH ppm 2.956 (3H, s, OCH3), 6.925 (s, N-H), 6.950 - 7.489 (13H, m, Ar-H), 13CNMR (75 MHz, CDCl3) δ ppm 50.02, 112.72, 120.28, 126.43, 126.80, 128.28, 128.99, 129.57, 129.25, 132.37, 132.58, 133.31, 144.19, 147.58, 148.79. HRMS (ESI): 376.1 calc. 376.84.
1-(4-methylphenyl)-3-(4-nitrophenyl)-5-phenyl formazan (Entry 18): UV (EtOH): λmax 404.21 nm (K-band), IR (KBr): ν cm−1 2968 (N-H str), 1648 (-C=C-), 1540 (-NO2), 410 (-N=N-), 1301 (-NO2), 1H-NMR (300 MHz, CDCl3): δH ppm 2.568 (3H, s, -CH3), 6.625 (1H, s, N-H), 6.653 - 8.240 (13H, m, Ar-H), 13CNMR (75 MHz, CDCl3): δ ppm 20.42, 77.43, 113.00, 115.31, 121.10, 123.56, 123.94, 124.03, 126.12, 127.88, 129.22, 129.92, 133.73, 141.76, 143.55, 146.81. HRMS (ESI): 360.12 (M+) obs, 359 (calc).
1-(4-nitrophenyl)-3-(4-nitrophenyl)-5-phenyl formazan (Entry 19): red, UV (EtOH): λmax 354.77 (K-band), IR (KBr): ν cm−1 2988 (N-H str), 1653 (-C=C-), 1548 (-NO2), 1418 (-N=N-), 1299 (-NO2), 1H-NMR (300 MHz, CDCl3): δH ppm 6.612 (1H, s, N-H), 6.64 - 8.174 (13H, m, Ar-H), 13C NMR (75 MHz, CDCl3): δ ppm 77.91, 113.66, 117.86, 121.82, 127.76, 128.43, 129.42, 130.00, 130.78, 136.08, 138.21, 147.85, 151.05, 152.08. HRMS ESI): 391.21 (M+ obs) 390 (calc.)
Acknowledgements
The authors are thankful to the HOD, Department of Chemistry, Gauhati University, for providing laboratory facilities.
NOTES
*Corresponding author.