1. Introduction
Zhumeria majdae Rech. f. Wendelbo (Lamiaceae) is a monotypic, relict species and endemic of Iran that is known by other names by native speakers: Mohrekhosh, Moukhash and Mourkhoosh. Although the Lamiaceae family has a cosmopolitan distribution, this plant is found only in a limited geographical range such as Geno where is a region with a unique mountainous terrain, which has located in southern Iran, northwest of the Persian Gulf port of Bandar Abbas [1] -[5] . The name of “Geno” means “smelly” in the local language, because its soil contains sulfur-containing compound, which may have effect the in vivo Mourkhoosh seeds germination percentage [6] -[8] .
Z. majdae is a medicinal plant which has long been used in Iranian traditional medicine as antispasmodic, anticancer, antileishmanial, antiplasmodial, antibacterial, antimicrobial, antioxidant properties and cytotoxic activities [9] - [18] . The leaves have been used for many years as a herbal tea, antiseptic and analgesic agent, curative for stomachaches, carminative especially in infants and for treatment of painful menstruation and dysmenorrheal [17] [19] .
In spite of its socio-economic importance and therapeutic benefits, it belongs to the species that is being threatened of disappearance and could be at a greater risk of extinction by the indiscriminate usage without due respect for conservation and/or regeneration. Indeed, the weakness of the in vitro regeneration of this species could be due to unknown limiting factors related to seed or environment, reduce considerably the chance of saving this endangered species. For example, the highest germination percentage of Z. majdae seeds that collected from Geno area was recorded only 10%in laboratory [20] [21] .
Thus, the major aim of this study was to investigate an efficient germination and an in vitro organogenesis for Z. majdae.
2. Materials and Methods
2.1. Plant Material
The seeds of Z.majdae were collected from Geno region, located in northwest of the Persian Gulf port of Bandar Abbas part of Hormozgan province in southern Iran. The mature seeds were identified by Ghahreman, a botanist of Tehran University (2009) and seed viability was determined by cut test.
The seeds were collected during the first week of May, were tested separately for their germination behavior. In vitro and in vivo seed germination tests were conducted at ambient temperature and in darkness, according to International Seed Testing Association guidelines (ISTA, 1993).
In in vivo germination test, the mature seeds were conducted to greenhouse on sandy loam soil that coming from Hormozgan province with Asmari-Jahrum (Paleocene-Miocene) Calcareous soils, EC = 31 - 71 mμ/cm, PH = 8.21 - 8.46, temperature = 0˚C - 50˚C, elevation = 600 - 2100 m properties.
For in vitro tests, First seed surface sterilization with 96% ethanol, commercial bleach (10% sodium hypochlorite), followed by washes with 70% Ethanol plus Tween 80 (Polyoxy ethylene sorbitan monooleate). Also, the seeds in each stage are rinsed with distilled water for 10 minutes and finally got them dry under room. At this stage, mucilage glaze was extracted from some seeds and they were too dried.
Five batches of 25 seeds were conducted in petri dishes (100 mm diameter, 15 mm deep) and placed on Murashige and Skoog (MS) medium (Murashige & Skoog, 1962). MS medium was supplemented with 30 g∙L−1 of sucrose and 7 g∙L−1 of agar, and pH was adjusted to 5.7 - 5.8 before autoclaving.
2.2. Seed Germination
The present experiment to study of different treatments effect on two group of seeds germination: 1) in vivo, 2) in vitro.
In the in vitro group, that were subjected to 5 treatments placed in culture petri dishes containing 52 ml of MS medium: Cold (48 h), NM (seeds without face the mucilage glaze extracted), 1M (face once the mucilage glaze extracted), 2 M (face twice the mucilage glaze extracted) and also The replications of 25 Seeds as a control (non-treated were placed on filter paper, moistened with distilled water) and kept on a laboratory bench at room temperature ((24 ± 1)˚C in dark)
Germination was expressed as percentage and as mean germination time (MGT) (Bonner, 1983). MGT was calculated by using the following equation:
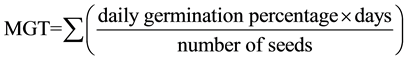
Non-germinated seeds at the end of the test were given a value of n + 1, where n = number of days in the test, and these values were included in calculation of MGT.
2.3. Callus Induction and Organogenesis
To do this experiment, first the explants (leaves, stems and roots) were washed thoroughly under running tap water for 15 - 30 minutes and also, rinsed with distilled water with few drops of dishwashing liquid1 for 5 - 10 minutes. Explants were sterilized with 80% Ethanol solution for 2 - 4 minutes and socked in commercial bleach (10% sodium hypochlorite) plus Tween 80 (0.05%) for 4 - 8 minutes and then rinsed three times with distilled water and conducted under vacuum.
The in vitro grown seedlings were aseptically excised in 5 × 5 mm2 pieces of leaves explants with approximately 0.5 cm long pieces of roots and stems explants and cultured on MS medium supplemented with different concentrations of 2-4-D(1 mg∙L−1) as a synthetic auxin, auxin indole-3-acetic acid (IAA)(0.1, 0.5 mg∙L−1) as a natural auxin, N-6-benzyladenine (BA)(1, 2, and 3 mg∙L−1) as a synthetic cytokinin and kinetin (Kin)(0.5, 1, and 3 mg∙L−1) as a natural cytokinin.
Explants of leaf, stem and root were then placed on the culture petri dishes containing MS mediumsupplementedwith7 different concentration and combinations of:
A: 2-4-D(1) + Kin(0.5) mg∙L−1
B: IAA(0.1) + Kin(1) mg∙L−1
C: IAA(0.1) + Kin(3) mg∙L−1
D: IAA(0.1) + BA(1) mg∙L−1
E: IAA(0.1) + BA(2) mg∙L−1
F: IAA(0.1) + BA(3) mg∙L−1
G: IAA(0.5) + BA(1) mg∙L−1
When culture media were being sterilized in an autoclave at 121.5˚C under 1.5 atm for 30 minutes and cooled to approximately 50˚C - 60˚C, kept them in the laminar airflow for 24 hours to avoid of contamination and then, the explants pieces were transferred to them.
2.4. Data Analysis
The experiments were carried out in completely randomized design were repeated four times and standard errors of the means were calculated. Data were subjected to analysis of variance using PASW (SPSS version 18). Duncan’s multiple range test (DMRT) (Duncan, 1955) was used to detect significant differences among the treatments with a probability of 95% (p ≤ 0.05). Germination percentage values were correlated using linear regressions (p = 0.05).
3. Results
3.1. Seed Germination
Results indicated the seeds belonged to 2M group had the highest germination percentage (40%) and the greatest velocity of germination (after 2 days of in vitro culture) in the duration of germination (85 days) but this group had low seedling length (3.3 cm). Although, NM group had the highest seedling length (4.75 cm) and the lowest germination velocity (after 24 days of in vitro culture) of the other groups and also, the germination percentage of this group reached to 25% in the duration of germination (50 days). However, the highest duration of germination related to 1M group compare to other groups (about 207 days), the germination percentage, the seedling length and velocity, respectively, were recorded 32.5%, 2.01 cm and 11 days.
The study of two cold groups indicated the germination duration and velocity, respectively, were recorded 45 - 50 and 14 - 17 days of in vitro culture) which of these groups had the lowest germination percentage (approximately 5% - 10%) of the other groups. Moreover, in the in vivo group were observed the percentage, duration and velocity, respectively, which is about 12.5%, 50 and 14 days after the culture (Figure 1).
3.2. Callus Induction
The maximum (Max) and minimum (Min) rate of callus induction were obtained from the root (R), leaf (L) and stem (S) explants on the MS medium supplemented with 7 different concentration and combinations(i.e., A)with 5 treatment-related (i.e., Control) as follows:
A: Max rate from R(missing data), L(45.5%, 2M), S(50%, Control) and Min rate from R(0, Cold and Control), L(8.3%, Cold), S(0%, Cold).
B: Max rate from R(58.3%, 2M), L(83.3%, 2M), S(83.3%, 2M) and Min rate from R(0, Cold and Control), L(20.4%, NM), S(2.6%, Cold).
C: Max rate from R(66.6%, NM), L(36.1%, NM), S(61.6%, 2M) and Min rate from R(0, Cold and Control), L(7.6%, Cold), S(1%, Cold).
D: Max rate from R(45.4%, 2M), L(58.3%, 2M), S(54.1%, 2M) and Min rate from R(0, Cold and Control), L(4.3%, Cold), S(0.6%, Cold).
E: Max rate from R(66.6%, 2M), L(72.2%, NM), S(66.6%, 2M) and Min rate from R(0, Cold and Control), L(6%, Cold), S(1.3%, Cold).
F: Max rate from R(56.6%, 2M), L(72.3%, NM) and S(66.6%, 2M) and Min rate from R(0, Cold and Control), L(5.3%, Cold), S(0.6%, Cold).
G: Max rate from R(83.3%, 1M), L(35%, NM) and S(53.3%, NM) and Min rate from R(0, Cold and Control), L(6.6%, Cold), S(1%, Cold).
3.3. Rooting
The in vitro regeneration of roots from leaf explants were recorded on the MS medium supplemented with 7 different concentration and combinations as this follows: C = 50%, D = 50%, E = 43%, G = 75% and no roots were observed on the others. Furthermore, rooting of root explants only were observed on MS medium supplemented with B = 15%, C = 42%, D = 16.6% and G = 16.6% concentration and combinations.
3.4. Leaf Formation
The in vitro regeneration of leaves from leaf explants were recorded on the MS medium supplemented with 7 different concentration and combinations as this follows: D = 25%, E = 50%, G = 8.33% and no leaves were observed on the others (Figure 2).
4. Discussion
Z. majdae as a medicinal plant along with its pharmaceutical industry properties is being threatened by the indiscriminate usage and limited distribution. Moreover, due to restricted seeds germination in the in vitro according to the past investigations [21] . Therefore, it is necessary to develop its tissue culture.
The seed germination of Z. majdae in the in vitro culture was indicated that the percentage and velocity of germination, respectively, were promoted and facilitated by the mucilage glaze extract. Although this effect was
![]()
![]()
![]()
![]()
Figure 2. Callus induction and organogenesis from root, stem, and leaf explants of Z. majdae(Stereomicroscope Figs): (A) Explants obtained in vitro of Z. majdae cultured on MS, ST: Shoot Tip (Scales); (B) Generation leaf of mass of callus from leaf explants, C: Callus, Le: Leaf explant, gL: generation Leaf; (C) Initial development of stem, C: Callus, Sh: Shoot explants; (D) Rooting stage from mass of callus, C: Callus, R: Rooting, hR: hairy Root.
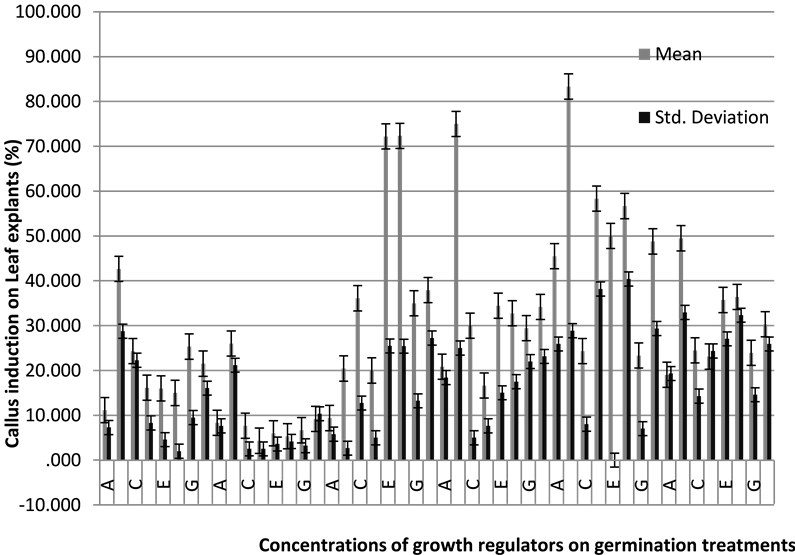
Graph 1. The effect of germination treatments and growth regulator combinations on the induction of callus from leaf explants.
not observed in the explants length and also, because of NM treatment had the highest seedling length and leaf number of the others, it is seems the mucilaginous material that may inhibited germination but facilitated seedling growth. In addition, 1M and 2M treatments had the highest duration on germination of the other groups but it is not clear whether this is the result of the mucilage glaze extract effect; that is, the duration on germination of 1M group was greater than 2M group.
It is noteworthy, the study of two cold treatments indicated the cold conditions had inhibited effects on all of the germination variables (e.g., the percentage, duration and velocity of germination), as well as callus induction and organogenesis except for callus induction from leaf explants in the MS + B. However, NM treatment had the lowest callus induction in this medium but reason is unclear.
The root explants of Z. majdae were induced callus in most of the hormone concentration, especially in the MS + G with 2M treatment. Moreover, the MS + B with 1M treatment were promoted callus induction on leaf and stem explants. Noticeably, 2M treatment was the best germination group for callus induction from root, stem and leaf explants of the others. However, callus induction from the leaves explants, was observed in all of the different concentration and combinations, by contrast of the stems explants. Thereby, the leaves and stems explants, respectively, showed great and limited totipotency in producing the callus induction in MS medium combined with different concentration and combination.
Based on these experiments, the low concentration of cytokinin has a negative effect on callus induction and also, it has positive effect on shoot production from leaf explants at intermediate concentration. Furthermore, high concentration of synthetic cytokinin and natural auxin promoted organogenesis. Because of the positive effect of auxin type and cytokinin concentration and also, inhibited effect of mucilage on callus induction, can be concluded, which is, at least in this part, cytokinin and auxin have neutralized the mucilage on the inhibition of callus induction.
Furthermore, the best rooting from root and leaf explants, respectively, were observed in the MS + C and MS + G. The maximum leaf formation of leaf explants were recorded in the MS + E.
In addition to the above factors, activated charcoal (AC) is another important factor for optimal tissue culture of Z. majdae, as Thomas, T. D also had pointed out the critical role of AC in plant tissue culture to improve cell growth and development [22] .
Eventually, based on data statistics and analysis, the effect of germination treatments and growth regulator combinations on the induction of callus are significant at 5% and also, there is an interaction effect between hormones dosage and germination treatments (Graph 1).
NOTES
1CH3(CH2)10CH2(OCH2CH2)nOSO3Na.