The Surface Reactivity and Electronic Properties of Small Hydrogenation Fullerene Cages ()
1. Introduction
The discovery of fullerenes in 1985 was the beginning of a new field of hydrogen storage research [1] . Fullerenes possess a wide range of applications in optical and electronic devices such as solar cells, photovoltaic and electro-optical devices [2] , in commercial cosmetic products [3] , as well as in biomedicine [4] . Hydrogen bond has been one of the most important elements bonded to the fullerene cages both inter- or intra-molecular [5] - [11] . Fullerene cages possess an outer and an inner surface available for hydrogen storage. Study the surface reactivity of endo-hydrogenation and exo-hydrogenation fullerene cages becomes an attractive research topic. Several hydrogenation techniques of C60 are well described. However, the structures and the symmetry of the small hydrogenation fullerene cages are not understood [12] - [14] . Therefore, the present work is investigated the surface reactivity and the electronic properties of endo-hydrogenation and exo-hydrogenations mall fullerene cages. The hydrogenation is applied with different concentrations and on seven different location sites of small fullerene cages.
2. Methodology
All calculations are performed with the DFT as implemented within G03W package [15] - [21] , using B3LYP exchange-functional [22] [23] and applying basis set 6 - 31 G (d, p). All obtained structures are fully optimized. In this work, the energy gap is calculated as Eg = ELUMO − EHOMO [24] , where ELUMO is the energy of the lowest unoccupied molecular orbital and EHOMO is the energy of the highest occupied molecular orbital. The hydrogen atoms are inserted inside (endo-hydrogenation) and outside (exo-hydrogenation) the fullerene cages. The reactivity and electronic properties of mono-hydrogenation (CnH) and fully hydrogenation (CnHn and CnHn+1) fullerene cages are investigated and then compared with un-hydrogenation (Cn) fullerene cages. There is only one hydrogenation site for C60 and C20 fullerene cages at  site and
site and  site respectively. However, there are four different hydrogenation sites for C40 cages at
site respectively. However, there are four different hydrogenation sites for C40 cages at 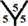 site,
site,  site,
site,  site and
site and 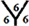 site and there are five different hydrogenation sites for C58 cages at
site and there are five different hydrogenation sites for C58 cages at  site,
site, 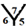 site,
site, 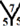 site,
site,  site and
site and 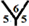 site, see Figure 1. The hydrogenation sites have been previously explained in details [13] .
site, see Figure 1. The hydrogenation sites have been previously explained in details [13] .
For the 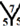 site, each angle of the pentagon is about 108˚ and each angle of the heptagon is about 128.57˚, so that the cone angle at the vertex of two pentagons and one heptagon is 344.57˚. In the
site, each angle of the pentagon is about 108˚ and each angle of the heptagon is about 128.57˚, so that the cone angle at the vertex of two pentagons and one heptagon is 344.57˚. In the  site, each angle of the hexagon is about 120˚, so that the cone angle at vertex of one heptagon, one hexagon and one pentagon is 356.57˚. In the
site, each angle of the hexagon is about 120˚, so that the cone angle at vertex of one heptagon, one hexagon and one pentagon is 356.57˚. In the  site, the cone angle at the vertex of two hexagons and one heptagon is about 368.57˚. For the
site, the cone angle at the vertex of two hexagons and one heptagon is about 368.57˚. For the 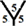 site, the cone angle at the vertex of the three pentagons is about 324˚. In the
site, the cone angle at the vertex of the three pentagons is about 324˚. In the 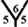 site, the cone angle at the vertex of two pentagons and one hexagon is 336˚. For the
site, the cone angle at the vertex of two pentagons and one hexagon is 336˚. For the ![]() site, the cone angle at vertex of two hexagons and one pentagon is 348˚. For the
site, the cone angle at vertex of two hexagons and one pentagon is 348˚. For the ![]() site, the cone angle at the vertex of three hexagons is equal 360˚, forming plane with zero curvature surface.
site, the cone angle at the vertex of three hexagons is equal 360˚, forming plane with zero curvature surface.
3. Results and Discussions
3.1. Hydrogenation Influence on the Electronic Properties of Fullerene Cages
3.1.1. Electronic Properties of Un- and Mono-Hydrogenation Fullerene Cages
The band gaps for un-hydrogenation (Cn) and mono hydrogenation (CnH) cages, from n = 20 to n = 60 are calculated and are listed in Table 1 and Table 2. In general, the hydrogenation increases the band gaps of the fullerene cages where Eg (CnH cages) > Eg (Cn cages). For mono hydrogenation, the band gaps of exo-hydrogena- tionfullerene cages are smaller than the band gaps of endo-hydrogenation fullerene cages. For exo-hydrogenation, the band gaps from C20H to C44H cages are in the following order: the Eg (![]() site) < Eg(
site) < Eg(![]() site) < Eg (
site) < Eg (![]() site) < Eg (
site) < Eg (![]() site). The band gaps of fullerene cages from the C46H to C60H cages are in the order Eg (
site). The band gaps of fullerene cages from the C46H to C60H cages are in the order Eg (![]() site) < Eg (
site) < Eg (![]() site) < Eg (
site) < Eg (![]() site) and for the C58H cage, the Eg (
site) and for the C58H cage, the Eg (![]() site) < Eg (
site) < Eg (![]() site) < Eg (
site) < Eg (![]() site) < Eg (
site) < Eg (![]() site) < Eg (
site) < Eg (![]() site). The smallest band gap is 0.94 eV for mono exo-hydrogenation C54H fullerene cage at
site). The smallest band gap is 0.94 eV for mono exo-hydrogenation C54H fullerene cage at ![]() site and is less than 1.36 eV of un-hydrogenation C54 fullerene cage.
site and is less than 1.36 eV of un-hydrogenation C54 fullerene cage.
![]()
Figure 1. Schematic representations of hydrogenation sites on small fullerene cages. The white circle refers to the location of hydrogenation carbon atom.
![]()
Table 1. The calculated energy gaps (Eg) for un-hydrogenation (Cn) and mono-hydrogenation (CnH) cages, from n = 20 to n = 60. Energy is given by eV.
![]()
Table 2. The calculated energy gaps (Eg) for un-hydrogenation C58 and mono-hydrogenation C58H fullerene cages. Energy is given by eV.
3.1.2. Electronic Properties of Fully Hydrogenation Fullerene Cages
The band gaps of the CnHn and CnHn+1 fullerene cages, from n = 20 to n = 60, are calculated and are listed in Table 3 and Table 4. The calculated band gaps of CnHn fullerene cages are higher than the band gap of Cn fullerene cages. The endo-hydrogenation CnHn+1 fullerene cages possess the same band gaps as Cn fullerene cages, however exo-hydrogenation CnHn+1 fullerene cages is reduced the band gaps of Cn fullerene cages form ~7 eV to ~5 eV.
Figure 2 shows the Mulliken charge populations for C54, C54H, C54H54 and C54H55 fullerene cages. For C54 cage the atomic population is almost uniform, about six electrons for carbon atom and one electron for hydrogen atom with small charge transfer about 0.024e, see Figure 2(a). For mono exo-hydrogenation C54H cage the charge transfer from hydrogen atom to carbon atom is increased to ~0.4e, see Figures 2(b)-(d). For the fully exo-hydrogenation C54H54 fullerene cage, the charge transfer from hydrogen atom to carbon atom is about 0.244e, see Figure 2(e), and for exo-hydrogenation C54H55 fullerene cages is ~0.2 eV, see Figures 2(f)-(h).
3.2. Hydrogenation Influence on the Reactivity Properties of Fullerene Cages
3.2.1. Surface Reactivity of Mono Hydrogenated Fullerene Cages
Going down from C60 cage to C20 cage by removing the C2 units, the number of hexagon rings is reduced and more pentagon rings are created. In other words, the number of hexagon rings is gradually reduced until is reached zero in case of C20 cage. To study the influence of hydrogenation on the surface reactivity of the fullerene cages Cn, the dipole moments are calculated for Cn and CnH cages, from n = 20 to n = 60 and are listed in Table 5 and Table 6. The Dipole moment is the measure of surface reactivity, where the high value of dipole
![]()
Table 3. The calculated energy gaps (Eg) of CnHn and CnHn+1 fullerene cages, n = 20 to n = 60. Energy is given by eV.
![]()
Table 4. The calculated energy gaps (Eg) of C58H58 and C58H59 fullerene cages. Energy is given by eV.
![]()
Table 5. The calculated dipole moments for un-hydrogenation fullerene Cn and mono hydrogenation CnH cages, from n = 20 to n = 60. The dipole moment is given by Debye.
![]()
Table 6. The calculated dipole moments for un-hydrogenation fullerene C58 and mono hydrogenation C58H cages. The dipole moment is given by Debye.
moment reflecting the high surface reactivity [25] [26] . First, it is found that the surface reactivity of mono hydrogenation fullerene cages (CnH) is higher than the surface reactivity of un-hydrogenation fullerene cage (Cn). Second, the surface reactivity for mono exo-hydrogenation fullerene cages is always higher than the surface reactivity of mono endo-hydrogenation fullerene cages. Third, the highest surface reactivity value is 4.11 Debye for the mono exo-hydrogenation C54H cage, comparing with 0.15 eV for un-hydrogenation C54 cage. Finally, the ![]() site is found to be the most reactive site. One can conclude that the surface reactivity of the mono hydrogenation fullerene cages is increased with increasing the number of pentagon-pentagon fusion, agrees with the previous observation [27] . Therefore, the less the cone angle, the larger the curvature and the highest reactive site. Result in the localized carbon atom at three pentagons has the highest reactive site, while the localized carbon atom at three hexagons has the lowest reactive site.
site is found to be the most reactive site. One can conclude that the surface reactivity of the mono hydrogenation fullerene cages is increased with increasing the number of pentagon-pentagon fusion, agrees with the previous observation [27] . Therefore, the less the cone angle, the larger the curvature and the highest reactive site. Result in the localized carbon atom at three pentagons has the highest reactive site, while the localized carbon atom at three hexagons has the lowest reactive site.
3.2.2. Surface Reactivity of Fully Hydrogenation Fullerene Cages
The surface reactivity for the CnHn and CnHn+1 fullerene cages is studied. The dipole moments for CnHn and CnHn+1 fullerene cages from n = 20 to n = 60 are calculated and are listed in Table 7 and Table 8. It is noticed that the surface reactivity for (CnHn+1) fullerene cage is higher than the surface reactivity for (CnHn) fullerene cage. Also, the surface reactivity of exo-hydrogenation fullerene cages is always higher than the surface reactivity of endo-hydrogenation fullerene cages. The order of the surface reactivity of endo-hydrogenation C58 H59 cage is at ![]() site >
site > ![]() site >
site > ![]() site >
site > ![]() site >
site > ![]() site and for the rest of the CnHn+1 fullerene cages is at
site and for the rest of the CnHn+1 fullerene cages is at ![]() site >
site > ![]() site >
site > ![]() site >
site > ![]() site. Finally, the highest surface reactivity value is found to be 0.34 Debye for the exo-hydrogenation C54H55 cage, comparing with 0.04 eV for C54H54 cage. From Tables 5-8 the dipole moments for Cn, CnH, CnHn and CnHn+1 cages, from n = 20 to n = 60, are calculated. One can sum-
site. Finally, the highest surface reactivity value is found to be 0.34 Debye for the exo-hydrogenation C54H55 cage, comparing with 0.04 eV for C54H54 cage. From Tables 5-8 the dipole moments for Cn, CnH, CnHn and CnHn+1 cages, from n = 20 to n = 60, are calculated. One can sum-
![]()
Table 7. The calculated dipole moments for CnHn and CnHn+1 cages from n = 20 to n = 60. The dipole moment is given by Debye.
![]()
Table 8. The calculated dipole moments for C58H58 and C58H59 cages. The dipole moment is given by Debye.
marize that the order of the surface reactivity is for CnH cages > Cn cages > CnHn+1 cages > CnHn cages. Also, the most reactive sites are found at ![]() site for CnH cages and at
site for CnH cages and at ![]() site for CnHn+1 cages.
site for CnHn+1 cages.
4. Conclusion
It is found that the surface reactivity order is for CnH cages > Cn cages > CnHn+1 cages > CnHn cages. Also, it is noticed that the smallest band gap is 0.94 eV for C54H cage when one hydrogen atom is exo-hydrogenatedat the ![]() site, comparing with 1.36 eV of un-hydrogenation C54 cage. In addition, the hydrogen adsorption inside the CnHn+1 fullerene cages does not affect the band gaps, however the hydrogen adsorption outside CnHn+1 fullerene cages reduces the band gaps form ~7 eV to ~5 eV.
site, comparing with 1.36 eV of un-hydrogenation C54 cage. In addition, the hydrogen adsorption inside the CnHn+1 fullerene cages does not affect the band gaps, however the hydrogen adsorption outside CnHn+1 fullerene cages reduces the band gaps form ~7 eV to ~5 eV.