Fundamentals on Thermodynamic Processes behind Clouds’ and Rainfalls’ Formation ()
1. Introduction
Clouds play a key role in the water and energy (irradiative, latent and sensible heat in this case) cycles. In low latitudes, about 62% of cloud cover is represented by low altitude clouds (consisting mainly of dry air melted with (gas-liquid) saturated water vapor), 32% by medium-altitude clouds (formed of dry air melted with (liquid- solid) saturated water vapor) and 6% by Cirrus (clouds formed of dry air melted with (gas-solid) saturated water). While the first reflecting more solar radiation and essentially cause cooling, the latter (low optical thickness and relatively transparent to sunlight) trap some of the Earth’s infrared radiation and substantially contribute to global warming. To better understand climate change and perhaps eventually eradicate some of their adverse effects, we must first learn about the clouds’ life cycle. Unfortunately, two aspects of this life cycle continue to be misinterpreted and that takes meteorologists away from understanding the true thermodynamic processes behind the formation of precipitation. Indeed, the prevailing idea so far about why the rainfall occurs was that after agglutination of water droplets with condensation nuclei, the size of the particle formed by the condensation nuclei connected with droplets of water increased considerably and caused its fall. This idea has led to numerous scientific publications [1] -[7] in which empirical distribution functions of meaningless clouds’ water droplets sizes were proposed. Estimates values provided by these empirical distribution functions, in most cases, were validated by comparison with UHF Radar measurements. The condensation nuclei concept has not been sufficiently exploited and this has led meteorologists to error, in their attempt to describe the clouds, thinking that clouds were formed by liquid water droplets. Indeed, MBANE BIOUELE paradox (2005) confirms this embarrassing situation. In fact, when applying Archimedes theorem to a liquid water droplet suspended in the atmosphere, we obtain a meaningless inequality 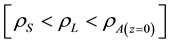 which makes believe that the densities of pure water in liquid and solid phases are much lower than that of the atmosphere considered at the sea level. This meaningless inequality is easy to contradict (e.g., emptying without any difficulty a bottle of pure liquid water on the ocean) but it allows to realize that clouds cannot be composed of suspended liquid (or solid) water droplets. Contrary to popular understanding, clouds are not formed by suspended liquid (or solid) water droplets: instead, clouds are earth’s atmosphere specific domains mainly formed by dry air
which makes believe that the densities of pure water in liquid and solid phases are much lower than that of the atmosphere considered at the sea level. This meaningless inequality is easy to contradict (e.g., emptying without any difficulty a bottle of pure liquid water on the ocean) but it allows to realize that clouds cannot be composed of suspended liquid (or solid) water droplets. Contrary to popular understanding, clouds are not formed by suspended liquid (or solid) water droplets: instead, clouds are earth’s atmosphere specific domains mainly formed by dry air 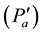 melted with saturated water vapor (esat). Evolution of gaseous particles that form the clouds, from gas phase to liquid or solid phases is possible only in the presence of condensation nuclei. Which means that: clouds generate rain only in the presence of condensation nuclei. In the nineteenth century, several laboratory experiments (unfortunately underused) already showed this water substance thermodynamics specifics. The chemicals needed for processing clouds’ aerosols into liquid or solid water particles had been baptized condensation nuclei by precursors such as William Henry Dines (1880) and Richerd Assmann (1884), who were two of the first studying microscopic mist suspended on mirrors (or well-clean and cold solid surfaces).
melted with saturated water vapor (esat). Evolution of gaseous particles that form the clouds, from gas phase to liquid or solid phases is possible only in the presence of condensation nuclei. Which means that: clouds generate rain only in the presence of condensation nuclei. In the nineteenth century, several laboratory experiments (unfortunately underused) already showed this water substance thermodynamics specifics. The chemicals needed for processing clouds’ aerosols into liquid or solid water particles had been baptized condensation nuclei by precursors such as William Henry Dines (1880) and Richerd Assmann (1884), who were two of the first studying microscopic mist suspended on mirrors (or well-clean and cold solid surfaces).
2. Processes behind the Formation of Clouds
In the past, researchers have often mentioned the presence of condensation nuclei in the atmosphere to trigger the fall of suspended liquid (or solid) water droplets contained in the clouds. This concept of condensation nuclei, at least empirically, has long moved away researchers from the true nature of Thermodynamics processes behind the formation of precipitation. Procedures closely related to the management of irradiative energy, latent and sensible heat flux (Figure 1).
2.1. Earth’s Atmosphere Particle Model
Atmospheric dynamics uses a very precise concept of particle (Figure 2) of air [8] -[24] . Namely:
- Few exchanges on molecular scale: one can follow a quantity of air which preserves certain properties.
- Quasi-static equilibrium: there is at any moment dynamic balance, the particle has the same pressure as its environment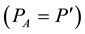 .
.
- No thermal balance: the heat transfers by conduction are very slow and are neglected. One can have 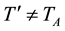
- The size of the particle can go from a few cm to 100 km according to the applications.
![]()
Figure 1. Regional variations in the coverage of high clouds (left) and low clouds (right) compared to the total annual average (in %), Statistic of Clima- tology covering 2003-2009.
![]()
Figure 2. Earth’s atmosphere particle model.
Taking into account the fact that the atmosphere is mainly composed of dry air and water vapor, Dalton’s law connects the pressure (PA) with the partial pressure of dry air (Pa) and water vapor (e).
(b) leads to Quasi-static balance equation
 (1)
(1)
(c) leads to possible temperature’s difference
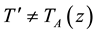 (2)
(2)
2.2. Clouds’ Formation Processes
According to thermodynamics Principles and laws, the necessary condition for a rain to occur is that the cloud forms. In other words, only part of clouds can produce rains. This therefore implies that water vapor contained in the moist air parcel shown in Figure 2 reaches saturated values. This process depends exclusively on pseudo isobaric cooling (e.g., cooling proceeded with eP quasi-constant) of the air parcel which brings esat(T) to eP. The dew point temperature (Td) is the temperature that gives exactly esat(T) = esat(Td) = eP on one of the three (3) Clausius-Clapeyron saturation curves (zesat), delivered in 1832. Once the dew point (Td) is reached, the further cooling below Td maintains eP equal to esat(Tp), water vapor within the parcel of moist air is then called saturated water vapor. The amount of steam which forms clouds closely depends on the beginning amount of temperature and humidity of air parcels which give birth to clouds, compared to water triple-point coordinates in eT-diagram [8] -[10] :
 (3)
(3)
Tcloud is cloud’s temperature; Td is air parcel’s dew point temperature (e.g., air parcels which gives birth to the related cloud). In the future, everyone must know that: clouds are earth’s atmosphere regions mainly composed of dry air 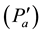 melted with saturated water vapor (esat).
melted with saturated water vapor (esat).
N.B.: only pseudo isobaric cooling (e.g., cooling at eP quasi-constant) gives birth to clouds. This means that the vertical speed (Vz) of the humid air parcel must exceed a critical value (VZ-min). This critical vertical speed is not well-known. A good example of speedy vertical motion is given by cloud associated with Tornadoes (Figure 3). Where Vz is so high that tornadoes related thermodynamic transformation is an adiabatic expansion typically associated with instantaneous high-cooling. That is why; clouds whose bases are located closely to the surface of the earth are formed. Tornadoes’ clouds look much more like the smokes rather than to a very tight grouping of suspended liquid water droplets.
3. Microphysics Structures of Clouds
Saturated water vapor has optical properties which depend on its temperature and concentration (or mole density). The advent of weather radars (Figure 4(a)) which are able to detect saturated water vapor particles, allows
![]()
![]() (a) (b)
(a) (b)
Figure 4. (a) Meteorological UHF Radar (b) hydro-aerosols sizes’ distribution proposed by 7 numerical models.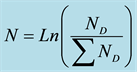 .
.
the publication of scientific articles on sizes’ (diameter in those cases) distribution of water aerosols that form clouds. These studies showed that clouds are for the most part mainly formed of very small hydro aerosols (diameter 0.05 cm) suspended in the atmosphere. The highest diameters (4b) of aerosols registered until now do not exceed around 0.7 cm.
4. Hydrostatic Equations
Let’s consider a water droplet (blue color) at rest, and not far from it, a portion of atmosphere with perfectly identical altitude and geometry to those of the water droplet. For each particle of water (blue Cubes on Figure 5) suspended in the atmosphere, the weight of the particle of water exactly compensates the vertical component of atmospheric pressure forces. Just not far from the particle of water at rest, the weight of portion of atmosphere with perfectly identical altitude and geometry to those of the water droplet compensates the same vertical component of atmospheric pressure forces (under Archimedes Theorem). In other words:
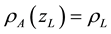 (4)
(4)
![]() (5)
(5)
Equations (4) & (5) show that hydrostatic balance is governed by the absolute equality between the density of the surrounding atmosphere and the density of the submerged body (water particles in this case). The volume of the particle plays no role in the conditions of hydrostatic equilibrium (only the density count). Knowing from many experiences that:
![]() (6)
(6)
Equation (6) leads to (7)
![]() (7)
(7)
Equation (7) leads to (8)
![]() (8)
(8)
According to Equation (8), the solid water particles are logically placed above the liquid water particles. This perfectly matches the meteorologists’ point of view.
5. Mbane Biouele Paradox
Archimedes theorem, applied to the Earth’s atmosphere, establishes that:
![]()
Figure 5. Hydrostatic balance of liquid (L) and solid (S) water particles in a meteorologically registered position.
![]() (9)
(9)
This meaningless inequality makes believe that the density of the liquid phase water is lower than that of the atmosphere at the sea level (z = 0). Equation (9) is completely false. Of course, if you empty a bottle of distilled water in the ocean (where z = 0), this water will not remain suspended in the air. For posterity and easy memorizing, Equation (9) has been baptized in the Ph./D 2nd thesis (2005) of its author: MBANE BIOUELE paradox.
6. New Descriptions of Clouds Considered as Rainfalls Systems
We now know that clouds are mainly made of dry air melted with saturated water vapor. If one divides member to member (4) and (5) by a reference density, these relationships will next compare the densities of hydro- aerosol, with the air density at different altitudes ZL and ZS
![]() (10)
(10)
![]() (11)
(11)
![]() (12)
(12)
where:
dg-L = density of water on (gas-liquid) saturation curve,
dg-s = density of water on (gas-solid) saturation curve,
dL-s = density of water on (liquid-solid) saturation curve,
dA = Air Atmosphere density at altitudes Zj-k (j and k are competing phases).
Both saturation curves are drawn in the phase equilibrium diagram published by Clausius-Clapeyron in 1832 (Figure 6). Considering hydro-aerosols instead of liquid or solid water droplets, hydrostatic equilibrium in terms of density of the suspended body in the atmosphere is expressed through equations (10) to (12). So, hydro- aerosol can leave the cloud and trigger rains only if it becomes a water liquid (or solid) droplet. For this to occur, it is necessary that hydro-aerosol meets a chemical that promotes its densification. Otherwise said, hydro- aerosols become liquid droplets and then fall as precipitation if they meet condensation nuclei. Chemicals that promote these thermodynamic mutations necessary for the formation of Precipitation may well be called condensation nuclei. We know from many laboratories’ experiences that some fine dust particles such as sulfur (emitted by volcanic eruptions) perfectly act as condensation nuclei. Other chemicals such as CO2 (emitted by many sources), spray (issued by the oceans) also act as condensation nuclei. Heavy rains cause irreversible damage. Therefore, the complete list of condensation nuclei must be established urgently by the scientific community to make it a little bit possible to effectively control certain harmful aspects of Climate Change. According to the water substance eT-plane, there are typically three (3) categories of clouds (Figure 7):
![]()
Figure 7. Clouds’ typical families and their localizing regions.
- The low-level clouds which pressure is given by ![]()
- The mid-level clouds which pressure is given by ![]()
- The high-level clouds which pressure is given by ![]()
N.B.: The two equal level surfaces of water vapor and temperature rating respectively at 6.11 mb and 0.0098˚C separate without any ambiguity parts of the troposphere that hosted each category of clouds (Figure 7).
6.1. Our Study Leads to Pertinent Properties on Clouds’ Vertical Motions
P1- There is no suspended liquid (or solid) water droplet in the clouds,
P2- If ![]() decreases
decreases ![]() will also decrease. i.e., low and high levels’ clouds should therefore move from (z) to (z - Δz). At the same time
will also decrease. i.e., low and high levels’ clouds should therefore move from (z) to (z - Δz). At the same time ![]() will increase and then Mid-level’s clouds move from (z) to (z + Δz).
will increase and then Mid-level’s clouds move from (z) to (z + Δz).
P3- If ![]() increases
increases ![]() will also increase. i.e., low and high levels’ clouds should therefore move from (z) to (z + Δz). At the same time
will also increase. i.e., low and high levels’ clouds should therefore move from (z) to (z + Δz). At the same time ![]() will decrease and then Mid-level’s clouds move from (z) to (z - Δz).
will decrease and then Mid-level’s clouds move from (z) to (z - Δz).
6.2. Our Study Also Leads to Pertinent Behaviors of Weather Phenomena [8] -[10]
Behavior (1)―Above the continents, esat (II) increase at night and then mid-level clouds gain altitude. This explains the birth of cold fronts (or squalls) which trigger (both night and day) cyclones when they crossing unstable atmosphere’s columns.
Behavior (2)―Above the oceans (oceans are excellent temperature regulators), clouds’ altitude remains fixed, both night and day. Therefore, cold fronts do not form over the oceans.
Behavior (3)―Atmosphere’s columns above hot surface is unstable areas. Mainly above hot oceans surfaces where temperature and humidity are important. That is one of the reasons why cyclones become mature when crossing hot oceans surfaces.
Behavior (4)―At the time of updrafts, clouds are easterly deflected (either Northern or Southern Hemisphere) by Coriolis. This explains why cyclones mainly move from East to West.
Behavior (5)―At the time of downdrafts, clouds are westerly deflected (either Northern or Southern Hemisphere) by Coriolis force.
7. Conclusion
This study opens new research avenues. Moreover, MBANE BIOUELE paradox clearly demonstrates that clouds are not composed of suspended liquid (or solid) water droplets. Indeed, when applying Archimedes theorem to a liquid water droplet suspended in the atmosphere, we have obtained a meaningless inequality
![]() which makes believe that the densities of pure water in liquid and solid phases are much
which makes believe that the densities of pure water in liquid and solid phases are much
lower than that of the atmosphere considered at the sea level. This meaningless inequality is easy to contradict (e.g., emptying without any problem a bottle of distilled water on the ocean) but it allows to realize that clouds cannot be composed of suspended liquid water droplets. Clouds are mainly composed of dry air mixed with saturated water vapor. Thermodynamics transformations which promote changes from hydro-aerosols to liquid (or solid) droplets are processed by catalyzers named condensation nuclei. Which means that: clouds generate rain only in the presence of condensation nuclei. In the nineteenth century, several laboratory experiments (unfortunately underused) already showed this water substance Thermodynamics reality. William Henry Dines (1880) and Richerd Assmann (1884) have shown great interest in studying microscopic mist suspended above mirrors. To summarize: there is no liquid (or solid) water droplet, suspended in the clouds. Indeed, all liquid (or solid) water droplets which are formed in clouds, fall under the effect of gravity and produce rains. This means that our current description of the clouds is totally wrong. In this study, we have described the clouds as a gas composed of dry air and saturated water vapor whose optical properties depend on temperature, i.e., when the temperature of a cloud decreases, the color of this gaseous system tends towards white.
Posthumous Acknowledgments
For their interest to thermodynamic processes mainly those behind clouds’ and rains’ formations in the nineteenth century, the author wants to acknowledge posthumously:
- Edmond Halley (1686)
- William Henry Dines (1880)
- Richerd Assmann (1884)
- Otto von Guericke
- Augustus Waller (1846)
- Coulier (1875)
- Clausius-Clapeyron (1832)
- Carnot (1824)