Methane Desorption from Homogeneous Distributed Micro-Pores of Coal ()
1. Introduction
Coal-bed methane, the major constituent of natural gas is mainly in the adsorption phase, solid or liquid state, in different pores of coal under under-earth reservoir conditions [1] -[3] . Methane existing in coal-pore will be desorbed for outer-disturbing, such as temperature, pressure or optic induced etc. [1] - [5] . The emission of flammable gases, predominantly methane from coal-beds with the increasing temperature, has always been a major problem for storage of coal. Meanwhile, methane has the highest H/C ratio, and consequently a higher research octane number than other fuels, which is a potential succedaneum for petroleum fuel. Coal-bed gas is also considered as potential causation leading to serious safety accidents in coal mines due to gas burst [4] [5] , and energy loss and global climate effect due to its emission. In addition to the coal-bed systems, many researchers have performed experiments to investigate the desorption kinetic process under pressure or temperature program in varieties of systems [6] - [10] . Their problem solving approach and the methodology are impressive, and would be applied to dealing with desorption of coal-bed methane.
For a long time, scholars have emphasized on the researches of coal-bed methane characteristics and have paid little attention to the desorption characteristics during coal-bed methane production [3] - [5] . The micro- mechanism of methane desorption with temperature program is key knot to evaluate the safety of piling coal. Simultaneously conducted theoretical analysis on desorption and adsorption processes, is the basis for a consideration of the mechanism of depositing of vapors and gases in the coal structure. However, few researches, especially taking the absorbed state of methane on the different positions, could be in good agreement with the experimental results.
The aim of the present study is to determine the desorption kinetic detail of methane by analysis of the desorption with temperature program, which provides further insight into the desorption mechanism. Although besides methane, ethane and carbon dioxide are present to a small extent in the seam gas, their dissolved fraction can be considered negligible compared to methane, and thus this study focuses on the absorption of methane solely.
In Section 2 we present the theoretical model and the simulation method and in Section 3, we give results of the simulations and make some discussions. Section 4 is the conclusion.
2. Theoretical Model and Simulation Method
To study the kinetic behavior of the desorption, many authors use Lattice gas model [11] - [13] which is a collection of atoms that may take on discrete positions. A configuration of the lattice is defined by site occupation variables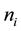 , where
, where 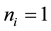 or 0 represents the site is occupied or not. The Hamiltonian of the model is
or 0 represents the site is occupied or not. The Hamiltonian of the model is
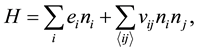 (1)
(1)
where 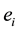 is the adsorptive energy of atoms, and
is the adsorptive energy of atoms, and 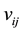 denotes the molecule interaction of the nearest neighbors. However, the structure of the coal-bed is very complicated. There are lots of micro-pores, meso-pores and macro-pores in the coal. The micro-pores in coal are estimated to have diameters ranging from 0.5 to 3 nm and occur as part of the coal matrix. In this article, we focus on the methane desorption mechanism from micro-pores, which can deposit several methane molecules. The methane is adsorbed in the pores at low temperature. As the temperature increasing, the molecules of the methane will be desorbed from the micro-pores. In order to study the kinetic process, we generalize the Hamiltonian by adding an on-site interaction:
denotes the molecule interaction of the nearest neighbors. However, the structure of the coal-bed is very complicated. There are lots of micro-pores, meso-pores and macro-pores in the coal. The micro-pores in coal are estimated to have diameters ranging from 0.5 to 3 nm and occur as part of the coal matrix. In this article, we focus on the methane desorption mechanism from micro-pores, which can deposit several methane molecules. The methane is adsorbed in the pores at low temperature. As the temperature increasing, the molecules of the methane will be desorbed from the micro-pores. In order to study the kinetic process, we generalize the Hamiltonian by adding an on-site interaction:
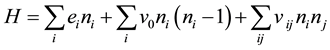 (2)
(2)
where 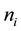 is the number of the molecules in the ith pore,
is the number of the molecules in the ith pore, 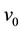 is the repulsive interaction when the molecules are in the same micro-pore and
is the repulsive interaction when the molecules are in the same micro-pore and 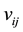 denotes the molecule interaction of the different micro-pores. If the distance of
denotes the molecule interaction of the different micro-pores. If the distance of
the adjacency pores is far enough, the interaction of the molecular is small. In this case, 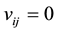 . In addition, we define the density of the molecular number,
. In addition, we define the density of the molecular number, 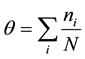 where N is the number of the micro-pores.
where N is the number of the micro-pores.
In our work, the Monte Carlo simulation is applied to analyze the detail of the desorption kinetic process of temperature program. For simplicity, we investigate one dimension chain. If the temperature is increasing with a constant rate, the probability for the desorption in temperature interval 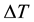 is,
is,
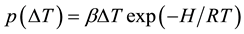
where 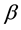 is a parameter related with the rate of the increasing temperature and the structure of the coal, and R is the universal gas constant. For the molecules may jump form one pore to another, we should give a jumping
is a parameter related with the rate of the increasing temperature and the structure of the coal, and R is the universal gas constant. For the molecules may jump form one pore to another, we should give a jumping
mechanism, which a molecular jump to its adjacency according to the probability 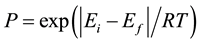 .
.
3. Results and Discussion
At first, it is considered that the interactions of different micro-pores might be very weak because of the long distant between them, therefore, we let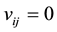 . The desorption kinetic process of methane molecules from the internal surface of coal was simulated with one dimensional chain during 200 - 460 K. We checked our theoretical model by tuning the absorption energy, from 40 kJ/mol to 60 kJ/mol to evaluate to the difficulty of the methane molecules with different energy at different point, as shown in Figure 1. The predicted desorption tem-
. The desorption kinetic process of methane molecules from the internal surface of coal was simulated with one dimensional chain during 200 - 460 K. We checked our theoretical model by tuning the absorption energy, from 40 kJ/mol to 60 kJ/mol to evaluate to the difficulty of the methane molecules with different energy at different point, as shown in Figure 1. The predicted desorption tem-
![]()
Figure 1. Homogeneous linear chain, variation without nearest-neighbor interaction energy.
peratures are basically in agreement with experimental one [14] , however, the desorption kinetic process behind the peak is extraordinary (abnormal).
The interaction between molecules, especially the jumping in the nearest-neighbor should be taken account in our model. If the condition of temperature or pressure changes, the kinetic energy of thermal vibration increases, making the gas molecule escape from the inner surface of the coal block by overcoming gravitation field and by becoming a free phase. The simulating results with jumping of nearest-neighbor indicated that it is reasonable, as shown in Figure 2. The methane structure in one-dimensional chain is a well-characterized adsorbate system, ideal for the study of fundamental surface desorption processes. It also constitutes the basis for the theoretical analysis of the thermodynamics of coal-sorbate systems and both enthalpy and entropy change in the adsorption process with respect to the specifics of coal structure.
To describe the desorption characters, we draw the desorption curve of e = 55 kJ/mol in detail with different temperature regions in Figure 3. The result shows that at low temperature section, which corresponds to the temperature region 210 - 230 K, there is no significant variation in desorption rate. The absorbing heating is lower than the desorption active energy of methane absorbed on the internal surface of the pore and fissuring of coal, that is that there an equilibrium of absorption and desorption. However, the vibration of the absorbed molecules on the internal surface will become increasingly sharpen for increasing absorbing heating and the collision of other molecules, meanwhile the diffuse rate will also increase for the increasing pressure in pore and fissuring of coal, which will cause a high exponential desorption kinetic process during 260 - 350 K. The probabilities of the velocity distribution of methane molecules at designed temperature also support the point, as shown in Figure 4. The molecules with high velocity will increase clearly with increasing temperature, the probability of collision will also be enhanced, then the desorption rate will also be increased.
Taking the coal sample as an example, the desorption energy of methane is taken 55 kJ/mol, then the desorption probability of it is obtained, as shown in Figure 5. The beginning temperature of desorption is room temperature, which consist with the desorption temperature in coal mine. The desorption rate increases violently over 360 K, which is extremely agreement with our previous experiment.
To identify the desorption difficulty of methane molecules at different energy point, the releasing probabilities at different temperatures were presented in logarithmic form, as shown in Figure 6. The desorption probabilities at 300 K, 350 K and 400 K, which have approximately same slope, are nearly similar behavior. Consequently, the choice of desorption will not be mainly effect with increasing temperature. However, the choice of desorption is clear at 200 K, that is those methane molecules with low desorption energy will easily be released at low temperature.
![]()
Figure 2. Homogeneous linear chain, variation with near-neighbor jump- ing interaction energy.
![]()
Figure 3. The desorption characteristic in detail with different temperature regions.
4. Conclusion
The models for one-dimension chain which is based on the jumping nearest-neighbor molecules is an appropriate method to discuss the kinetic process of thermal desorption of adsorbed molecules on the internal surface of coal. The thermal desorption mechanism is different from the pressure one. Analyzing the desorption curves of different adsoptive energy, we find that the initial desorption temperatures can be strongly affected by adsorp-
![]()
Figure 4. The velocity distribution of methane in lattice with different temperatures.
![]()
Figure 5. The desorption probabilities of methane with desorption en- ergy, 55 kJ/mol.
![]()
Figure 6. The choice of methane with different desorption energy.
tion energy of molecules. In addition, the desorption probabilities shows that, at low temperature, the desorption molecules is of low energy, however, for the virbration and collision of other ambient molecules increasing, the energy of the molecules desorbed from coal is much higher in high temperature. Although the conclusions which are agreement with the experiment, are get in the article, for the coal-bed is of very complicated structure, one-dimension micro-pore chain could not describe the system precisely. Hence, more complex models, such as 2D/3D structures, complex networks and some heterogeneous structure in different dimensions, are needed to be further studied.
Acknowledgements
The work is supported by the Natural Science Foundation of China under Grant No. 11405127 and Scientific Research Program Funded by Shaanxi Provincial Education Commission Program No. 12JK095.
NOTES
*Corresponding author.