Bulk Density in Jack Bean’s Development Grown in Cerrado Oxisol ()
1. Introduction
Soil compaction is a major problem regarding quality of soil and development of sustainable agriculture, because it modifies water and airflows in the soil reducing the productivity of agricultural crops [1] .
The increased bulk density is directly related to difficulties of roots in overcoming soil’s mechanical resistance [2] , causing changes in the growth and development of plants due to the difficulty the roots have in the absorption of nutrients in the soil [3] .
The reduction in aeration and water availability in the soil due to a decrease in porosity caused by soil compaction causes a decrease in crop yields [4] .
In intensive uses of the soil, there is the presence of compacted layers in soil subsurface, known as “grid walk”, causing an excessive increase of bulk density that results in decrease of the total volume of pores, thus reducing permeability and water leakage, aggregates breakdown and increase in penetration resistance, causing physical damage to soil quality [5] .
Plant’s growth is directly reduced by the mechanical resistance that the ground offers to root growth and it increases with an increasing bulk density [6] . An alternative to alleviate this problem is the use of species with a deep and vigorous root system [7] .
The use of green manure as a conservation practice has been recommended for it provides significant benefits to agriculture. The advantages of using green manures were verified for soil protection, reduction of nutrient losses due to erosion control, which ensures gain of organic mass, recovery and recycling of nutrients and nitrogen supply, especially when using legumes [8] [9] .
The use of green manure is an alternative practice to ease the problem of compression, because its root system creates spaces that have an importance for gas exchange, water infiltration and improving soil’s physical conditions, thereby creating a more favorable environment for root growth [10] .
Several plant species can be used for green manure, but the preference for legumes is higher due to numerous advantages to soil unpacking because of its pivoting system and the possibility of biological nitrogen fixation.
Canavalia ensiformis, popularly known as jack bean, is a legume widely used in the Southeast and South of Brazil, planted in intercropping between the hills of annual or perennial crops, and in rotation before or after the annual crop [11] .
Green manure with jack bean is a practice that has been adopted in order to increase soil fertility mainly through biological nitrogen fixation [12] .
Taking this in consideration, the objective was to evaluate the effect of bulk density in the development of jack bean (Canavalia ensiformis) in Cerrado Oxisol.
2. Material and Methods
The experiment was conducted in a greenhouse at the Federal University of Mato Grosso, Rondonópolis-MT, in the period from June to September 2013. The soil used was a Oxisol from an area with Cerrado vegetation, collected at the 0 - 0.20 m layer. Its chemical and particle size characterization was performed according to [13] and had the following characteristics: pH (CaCl2) = 4.1; exchangeable Al (cmolc∙dm−3) = 1.1; Ca (cmolc∙dm−3) = 0.3; Mg (cmolc∙dm−3) = 0.2; P (Mehlich) (mg∙dm−3) = 2.4; K (mg∙dm−3) = 28; S (mg∙dm−3) = 6.8; organic mass (g∙dm−3) = 22.7; V (%) = 6.5; clay (g∙kg−1) = 367; sand (g∙kg−1) = 549; silt (g∙kg−1) = 840.
The average maximum and minimum temperature during this period was 37.3˚C and 22.6˚C respectively, showing thus a 29.9˚C average. The mean maximum and minimum relative moisture was 88.2% and 31.7%, respectively, watching an average relative moisture of 60%.
Soil pH was adjusted with the addition of dolomitic limestone, increasing base saturation to 60%. The basic fertilization was performed prior to compression of the experimental unit, in solid and granular forms, with 80 mg∙dm−3 K2O (potassium chloride) and 150 mg∙dm−3 P2O5 (superphosphate). There was no nitrogen fertilization because jack bean is a legume that has good efficiency in fixation.
Five jack bean the common cultivar seeds were sown in the experimental unit (Figure 1(a)) and, at the 8th day, there was the thinning, leaving two plants per experimental unit (Figure 1(c)).
The experimental unit was represented by a pot with 5.28 dm3, made with PVC pipe with 150 mm internal diameter and 300 mm in height, comprised of three 100 mm rings joined with “silver tape” adhesive. The upper and lower rings were filled with 1.0 Mg∙m−3 density soil, while the intermediate rings were subjected to 1.0, 1.2, 1.4, 1.6 and 1.8 Mg∙m−3 compression levels (Figure 2). At the bottom of the experimental unit, a 1 mm polyethylene mesh was used to close the base of the pots, which was affixed with a rubber obtained by a cross-section of an automotive air chamber [14] .
![]() (a) (b) (c) (d)
(a) (b) (c) (d)
Figure 1. Development of jack beans at four, eight, twelve and twenty days, respectively ((a); (b); (c) and (d)).
![]()
Figure 2. Representation of an experimental unit, showing the position of the 0.10 m compacted layer.
To determine the soil weight to be used for each density level, Equation (2) was used.
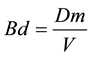 (1)
(1)
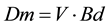 (2)
(2)
where:
Bd―Bulk density (Mg∙m−3);
V―Cylinder volume corresponding to the 0.10 m layer (1.767 m−3);
Dm―Dry soil mass (Mg).
The ideal compaction moisture at 16% soil mass basis was established by previous trials conducted in a Proctor test laboratory [15] . The soil mass placed on the ring to be compressed was determined by the Equation (3).
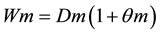
where:
Wm―Went soil mass (g);
MSS―Dry soil mass (g);
θm―Soil moisture-based mass (g∙g−1).
Soil compaction was carried out using a Bovenau P15ST hydraulic press (Figure 3). Plastic dishes were used in the back of experimental units to assist in irrigation by capillarity [16] .
The experiment was conducted in an entirely randomized design with five levels of compression and five replicates (Figure 4).
The results were submitted to variance analysis and, when significant, to regression analysis, both with a 5% probability, using SISVAR software [17] .
Irrigation was performed on the surface of the pots until the establishment of the plants and, from 15 days, soil moisture was kept by capillarity, adding water to the dishes under the pots, seeking to provide the plants the need to surpass the obstacle of the compacted subsurface layer in search of water and nutrients [15] .
At 60 days after sowing, at the beginning of flowering, the cutting the plants close to the ground and the root system washing were made evaluating the variables from the SPAD index (Soil Plant Analysis Development)
![]()
Figure 3. Soil compaction using a Bovenau P15ST hydraulic press.
![]()
Figure 4. Overview of the experiment with jack beans grown in different bulk density levels in a greenhouse.
determined by a chlorophyll clorofilog model CFL 1030 electronic meter with an average reading of three leaves per pot. Plant height was measured with a graduated ruler (cm) and the diameter with a digital caliper. To determine dry mass, the collected plant material was placed in an identified paper bag and then dried in an forced-air circulating oven at 65˚C for 72 hours [18] , and then weighed with a precision scale.
3. Results and Discussion
All variables fitted the linear regression model, with significant effects on bulk density levels.
For the height of jack bean plants, there was a significant response to bulk density. A significant decrease was observed in plant height with the increase of levels of density to 54.50% (Figure 5(a)), where the increase in bulk density caused a restriction of jack bean plant height (Figure 5(b)). Studying bean plants (Phaseolus vulgaris L.) in Ultisol Oxisol, [19] stated that plant height is an important indicator to assess the effects of soil compaction in physiological processes of plants.
![]() (a)
(a)![]() (b)
(b)
Figure 5. Number of leaves of jack bean plants according to levels of bulk density. ***Significant at 0.1%. (a) Experimental unit of jack bean plants in soil densities 1.0, 1.2, 1.4, 1.6 and 1.8 Mg∙m−3, respectively (b).
The results observed in this study for the variable plant height of jack beans are in agreement with those found by reference [20] , in which there was a significant decrease in plant height of pigeon pea beans (Cajamus cajan) with increasing levels of compression, with a reduction between the lowest and the highest level of bulk density of 51.07% at 33 days after emergence.
Plant height results in this study did not corroborate those observed by reference [21] , who, studying soybean cultivars (Glicine max L.), found that the density of the soil did not influence plant height.
The number of jack bean leaves decreased by 58.07% when comparing the absence of compression (1.0 Mg∙m−3) to the maximum compression level (1.8 Mg∙m−3) (Figure 6).
These results are consistent with those observed by reference [14] , who found that the levels of soil densities might interfere with the availability of nutrients to roots, contributing negatively on nutrient absorption and reducing leaf production.
The results observed in this study show that, as bulk density increases, the root system has less access to water and nutrients, causing a smaller development of shoots. Reference [19] also found that soil compaction affects the development of bean leaves.
As to stem diameter, significant reductions were observed due to the increase in bulk density levels with a decrease of 26.21% (Figure 7). The study of stem diameter is important because plants with a greater stem diameter have a greater balance in the growth of shoots [22] .
![]()
Figure 6. Number of leaves of jack bean plants according to levels of bulk density. ***Significant at 0.1%.
![]()
Figure 7. Stem diameter of jack bean plants according to the levels of bulk density. ***Significant at 0.1%.
Stem diameter results corroborate those found by reference [20] , who observed reductions in pigeon pea stem diameter due to increased levels of soil densities.
Bulk density levels (Figure 8) influenced the dry mass of jack bean leaves. Thus, it can be seen that, with the increase in bulk density, there was a 31.80 g decrease in dry mass yield of leaves between the lowest (1.0 Mg∙m−3) and the highest bulk density (1.8 Mg∙m−3), with a reduction equivalent to 80%. Thus, it can be inferred that, as an increase in bulk density happened, there was a restriction of water and nutrients absorption by the plants due to physical constraints of the soil.
This reduction in shoot dry mass was explained by reference [23] working with six compaction levels in a Entisol, stating that the decrease occurred in function of changes in soil physical properties caused by levels of bulk density, thereby decreasing the absorption of nutrients and carbon accumulation by photosynthesis.
Studies by reference [3] , working with five levels of soil densities (1.0, 1.2, 1.4, 1.6 and 1.8 Mg∙m−3) in a soil collected in the same area as that of this study, found that the physical limitation of the soil decreased by 37.77% the dry mass of leaves between the highest and the lowest soil densities.
Stem dry mass also decreased by 79.80% with the increase in bulk density (Figure 9). The increase in bulk density reduced the development of shoots with the decrease in number of leaves, height and dry mass of leaves
![]()
Figure 8. Dry mass of leaves of jack bean plants according to levels of bulk density. ***Significant at 0.1%.
![]()
Figure 9. Dry mass of stem of jack bean plants according to levels of bulk density. ***Significant at 0.1%.
and stem. In this context, it can be hypothesized that plant roots grown in compaction stress conditions send signals to shoots to reduce growth rate [24] .
The dry mass of jack bean roots (Figure 10) showed a reduction when compared to the absence of compression (1.0 Mg∙m−3), with the highest level of bulk density (1.8 Mg∙m−3), showing a decrease of 27.33%.
Root dry mass is an important variable to assess the growth and development of a culture. In this sense, [7] found that the declivity of jack bean growth curve indicates that the roots of this species are susceptible to increased compression because morphological and physiological changes more significantly decrease the growth of plants.
Root development was affected by bulk density at 1.4 and 1.6 Mg∙m−3 densities (Figure 11). In these treatments, only some roots exceeded the compacted layer, reaching the lower ring where they developed with the formation of nodules. However, at a 1.8 Mg∙m−3 density, the roots could not overtop the compacted layer. Therefore, their growth and development was limited in the upper ring. Similar results were found by reference [25] , who found that in some pots with 1.44 and 1.75 Mg∙m−3 soil densities plants forced passage through a minimum gap between the iron cylinder and the pot; a single root penetrated through the gap, reaching the lower third and branching out considerably.
![]()
Figure 10. Dry mass of roots of jack bean plants according to the levels of bulk density. ***Significant at 0.1%.
![]()
Figure 11. Roots of jack bean plants in soil densities 1.0, 1.2, 1.4, 1.6 and 1.8 Mg∙m−3, respectively.
Among the factors previously mentioned, the main reason for reduction or modification of physiological functions of the roots, associated with the smaller volume of soil explored by them under compaction conditions, is poor nutrition of plants, so that they could not maintain the same growth pattern as observed for the lowest compression [7] .
Reference [26] observed similar results with soy, where soil compaction decreased growth and root development of plants, thereby decreasing the area explored by the roots.
The number of nodules and dry mass of jack bean nodules had a significant effect on soil compaction factors. Bulk density levels represented an 87.42% decrease in the number of nodules and 93.26% in dry mass of nodules (Figure 12(a) and Figure 13). The number of nodules is directly related to root dry mass (Figure 12(b)).
Similar results were obtained by reference [20] , who observed a reduction in the number of nodules in pigeonpea with increased bulk density levels.
Reference [27] , in a study on the influence of soil compaction in soybean, observed that the increase in bulk density affected the accumulation of biomass of the root system. According to the author, it is possible that this effect has involved the reduction of the number of nodules, since they are associated with the root system of the plant. The greater the root system, the higher the number of nodules.
There was also the absence of nodules in compacted layers at 1.4 Mg m-3 density level (Figure 14). These results are in agreement with those found by reference [25] with soybean grown in Oxisol 1.44 Mg∙m−3 and 1.75 Mg∙m−3 bulk density levels caused structural changes in roots, preventing the formation of nodules.
At the SPAD index reading, there was an adjusted linear regression model based on soil densities (Figure 15). A 21.32% decrease between the lowest density (1.0 Mg∙m−3) and the highest density (1.8 Mg∙m−3) was observed. This difference is due to the possible reduction of chlorophyll concentration promoted by the reduced availability of total nitrogen in the tissues: higher levels of soil densities compromise the absorption of this nutrient by the plant.
![]() (a)
(a)![]() (b)
(b)
Figure 12. Number of nodules of jack bean plants according to the levels of bulk density. ***Significant at 0.1%. (a) Number of nodules of jack bean plants according to the levels of bulk density (b).
![]()
Figure 13. Dry mass of nodules of jack bean plants according to levels of bulk density. ***Significant at 0.1%.
![]()
Figure 14. Partial view of a jack bean pot with a 1.4 Mg∙m−3 bulk density.
![]()
Figure 15. SPAD readings of jack bean plants according to levels of bulk density. ***Significant at 0.1%.
SPAD readings measure the chlorophyll content in plant leaves, but it is also an information tool to identify nitrogen nutrition deficiency, since there is a positive correlation between SPAD readings and the concentration of nitrogen in leaves [28] - [30] .
Studies conducted by reference [31] with Cerrado Oxisol with bulk density levels posit that the decrease in chlorophyll content may be related to the decrease in root mass, thereby decreasing the absorption of nitrogen by plants.
4. Conclusion
The jack bean suffers restrictions on development in morphological and productive characteristics with the increase in soil density levels, showing little efficiency as a decompacting plant in Cerrado Oxisol.
NOTES
*Corresponding author.