Effects of Process Parameters on the Size of Nanostructure Magnesium Oxide Synthesized by a Surfactant and Ligand Assisted Wet Chemical Method ()
1. Introduction
Metal oxide nanomaterials exhibit unique physical and chemical properties and have potential applications in magnetism catalysis, electro optical devices, sensors, and nanodevices [1] - [3] . Magnesium oxide is suitable for insulation applications due to its low heat capacity and high melting point [4] . MgO is used as a fundamental material for chemical pumps [5] , and as a substrate material for the epitaxial growth of thin films with desired electronic or magnetic properties [6] . MgO-thin films have been proposed for use as protective coating on phosphor screens [7] and as a substrate for carbon nanotubes growth [8] . Nano-sized MgO can be used for relative adsorbents. They have been used for the decontamination of chemical warfare agents [9] , reducing cholorofluoro carbons [10] . Wet chemical is a method for the synthesis of nanostructure MgO. Various morphologies nanostructures of MgO, including nanowires, branched nanostructures nanotubes and nanocubes, have been obtained [10] . Over the past few years, synthesis methods have been developed for the preparation of nanocrystalline MgO, including sol-gel [11] [12] , hydrothermal [8] , laser vaporization [9] , chemical gas phase deposition [10] , combustion aerosol synthesis [11] , and precipitation [13] .
In this paper, we have described a simple, economical, and moderately low temperature (500˚C) solution for the preparation of MgO nanopowder. The decomposition of the Mg(OH)2 intermediate phase in the presence of PVA and PEG surfactants gave highly monodispersed MgO particles, with a crystal size of less than 15, 15 - 40 and more than 40 nm.
2. Experiment
Materials: magnesium acetate butahydrate Mg (CH3COO)2.4H2O, poly ethylene glycol (PEG; MW: 6000), dimethyl solfoxide (DMSO), sodium hydroxide (NaOH), ethanol (C2H5OH), methanol (CH3OH), citric acid (C6H8O7), sorbitol (C6H14O6), 4-methyl morpholine and EG (C2H6O2), EDA (di ethylene amine monohydrate), oleic acid (C18H34O2), and poly vinyl alcohol (PVA; MW: 72000) were purchased from Merck and used without further purification. De-ionized water was used throughout this study.
Synthesis: Nanostructure MgO was produced using a wet chemical method. For synthesis of MgO nanoparticles, 10.723 g Mg (CH3COO)2∙4H2O and 50 ml oleic acid were dissolved in 100 ml of H2O (de-ionized water) under vigorous stirring conditions at 25˚C. Then while stirring, a mixture of NaOH 4M and 2 g PEG 6000 was added dropwise to the solution at room temperature until a pH of 10 was reached. After being vigorously stirred, the final white solution was kept at a temperature of 65˚C for four hours. After precipitation, the solution was stirred for another two hours and then filtered and washed several times with deionized water and alcohol. It was finally dried at 85˚C in air overnight and calcined in air at 500˚C for five hours using a muffle furnace.
The experiment was carried out again using 2 g PEG 6000 and 50 ml sorbitol ligand in the 100 ml CH3OH solvent, 20 g citric acid ligand for strong citric acid in the presence 100 ml H2O solvent and 2 g citric acid ligand in the presence 100 ml H2O solvent, 2.4 g tri Phenyl Phosphine ligand in the presence 100 ml EG solvent. The experimental procedure was the same as the above synthesis.
To synthesize a sample with poly vinyl alcohol surfactant, first 2 g PVA 72000 was dissolved in 100 ml water at 90˚C under vigorous stirring to form a transparent solution. The experimental procedure was the same as the above synthesis.
The experiment was carried out again using 2 g PVA 72000 and 50 ml sorbitol ligand in the 100 ml H2O solvent, 2 g PVA 72000 and 50 ml EDA ligand in the presence of 100 ml ETOH solvent, 2 g PVA 72000, and 50 ml 4-methyl morpholine ligand in the presence of 100 ml H2O solvent. The experimental procedure was the same as the above synthesis.
Characterization: The morphology and the crystalline structures of the as-grown products were characterized by Field Emission Scanning Electron Microscopy (FESEM, Hitachi S4160 Cold Field Emission, Japan), Transmission Electron Microscopy (TEM, Model EM 900, Company Zeiss) and X-ray diffract meter (XRD, diffractometer using the Cu K2a source at 40 kV and 40 mA).The infrared spectrum of the MgO was measured by a Perkin-Elmer spectrum RXI FT-IR spectrometer. The surface area was measured by being calcined in air at 500˚C for five hours (BET, Belsorp mini II, Japan).
3. Results and Discussion
Figure 1 represents the FESEM images of MgO calcined at 500˚C and Mg (OH)2 in the PEG 6000 and oleic acid ligand, the PVA 72000 and oleic acid ligand, and the PVA 72000 and EDA ligand, respectively. From the FESEM observation, the Mg(OH)2 precursor contains surfactant PEG 6000 with plate-like morphology, and the
![]()
Figure 1. The FESEM images of MgO in: (a)-(c) PEG 6000 and oleic acid, PVA 72000 and oleic acid, PVA 72000 and EDA at 500˚C. The FE-SEM images of Mg(OH)2 in: (d)-(f) PEG 6000 and oleic acid, PVA 72000 and oleic acid, PVA 72000 and EDA. The TEM images of MgO in citric acid (g), PVA 72000 and EDA (h).
produced MgO shows a different morphology (sphere-like). The Mg(OH)2 precursor contains surfactant PVA 72000 and an oleic acid ligand with a plate-like morphology, and the produced MgO shows a different morphology (sphere-like). However, the Mg(OH)2 precursor contains surfactant PVA 72000 and an EDA ligand with a sphere-like morphology, and the produced MgO also shows the same morphology. The effects of the ligand and surfactant on the size of nanostructure magnesium oxide show that changing the surfactant from PEG to PVA resulted in a decrease in the particle size (from an average of 40 nm to 12 nm). The results showed that adding PVA has a significant effect on the structural properties and increased the specific surface area. These images clearly show homogeneous nanostructures as well as remarkably different conditions. We have observed an agglomeration of particles in all the cases due to prolonged reaction time [14] [15] . The agglomeration effect was prominent in aqueous solvent compared to organic solvents [16] . A particle size of 8nm was obtained in PVA, diethylene amine and ETOH. According to this report, the surfactant, together with the ligand, played an important role in determining the morphology and size of the products. For systematic transformation, a series of controlled experiments was carried out. First, we regularly changed the reaction surfactant while other reaction limits were kept constant. As can be seen in Figure 1(a) and Figure 1(b), the morphology was not changed but the particle size of the MgO was reduced by changing the surfactant from PEG 6000 to PVA 72000.
The TEM images of MgO calcined at 500˚C is shown in Figure 1. As can be seen, both the samples show a nanocrystalline structure with a spherical shape. Figure 1(g) shows the TEM topography of nano-sized MgO with a length of 22 nm and a breadth of 25 nm. Figure 1(g) also shows the layered structure of MgO. The spherical shape of nano-sized MgO is shown in Figure 1(h). With a length of 12 nm and a breadth of 16nm, the sphere-like particles are presented without any agglomeration.
Several factors, including the surfactant and the ligand, have an impact on the particle size and surface area. The particle size of MgO increased rapidly when the surfactant was changed from PVA 72000 to PEG 6000.
The mechanism of MgO is usually accepted as follows:
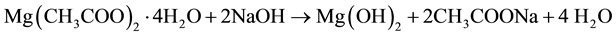
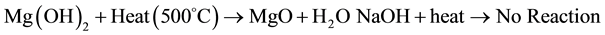
There are many separated colloidal Mg(OH)2 clusters in the preformed suspension, some of which can act as the nuclei for MgO growth. The existence of hydroxyl groups limits the growth of MgO nuclei [17] [18] .
The investigation into the effects of surfactant and ligand on the morphology and crystal size of the samples, as illustrated in Figure 2(a) and Figure 2(b), show that changing the amount of citric acid ligand from 2 g to 20 g, when other reaction parameters are kept constant, increases their size. Figure 2(c) and Figure 2(d) show that
![]()
Figure 2. The FE-SEM images of MgO at 500˚C: (a) citric acid; (b) strong citric acid; (c) PEG 6000 and sorbitol; (d) PVA 72000 and sorbitol; (e) PVA 72000 and 4-methyl morpholine; (f) EG.
changing the surfactant from PEG 6000 to PVA 72000, when other reaction parameters are kept consonant, reduces its size. Figure 2(d) and Figure 2(e) also show that changing the ligand from sorbitol to 4-methyl morpholine, when other reaction parameters are kept constant, changes the morphology from nanospherical particle to nanocube particle.
XRD: The crystal structure of the MgO sample (Figure 3) was measured from the X-ray brodline by using the Scherrer equation:
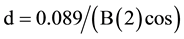
where B(2q) is the width of the XRD pattern line at half peak-height (rad), l the wavelength of the X-ray, q the angle between the incident and diffracted beams, and d the crystal size of the powder sample (nm) [19] [20] . The X-ray diffracts gram shows two sharp lines. The other crystal planes were suppressed and confirmed the semi- crystalline nature of MgO. The appearance of the d crystal plane authenticated the structure of the MgO hexagonal-like structure. In this investigation, our aim was to prove that MgO synthesized by the one-pot method is nano sized with a layered structure.
N2 adsorption isotherm: The specific surface (SA) of MgO nanoparticles is determined by a BET method. The theoretical size of the particles was also calculated from the following equation:
![]()
![]()
Figure 3. The pore diameter distribution, N2 adsorption and a BET plot ((a), (b), (c)) in the PEG 6000, and ((d), (e), (f)) in the PVA 72000 respectively, (g, h) The wide-angled XRD patterns of (g) MgO in PVA 72000, (h) MgO in citric acid.
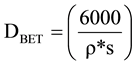
where DBET is the equivalent of the particle diameter in nanometers, ρ is the density of the material in g∙cm−3 and S is the specific surface area in m2∙g−1.
Figure 3 shows the distribution curve of pores size, the isotherm of corresponding nitrogen adsorption/de- sorption, and the BET surface area plot of the produced MgO. The distribution of pores volume with their radius is calculated by the BJH method. The MgO samples show relatively narrow pore size distributions. Their average pore sizes were in the range of 13.66 - 14.57 nm, the average particle sizes fell into the range 34.6 - 12.22 nm, the average pore volume fell into the range (v p) 0.29 - 0.57 m3∙g−1, and the average surface area fell into the range 48.8 - 136.95 m2∙g−1 for the surfactant PEG 6000 in the presence of a sorbitol ligand and PVA 72000 in the presence of an EDA ligand, respectively. According to the IUPAC classification, the N2 isotherm is an isotherm type III with a large type H3 Hysteresis loop. This type of hysteresis is usually found in solids consisting of aggregates or agglomerates of particles forming slit-shaped pores with no uniform size and/or shape. The nitrogen adsorption method has been used to determine the BET surface area and the pore size distribution of typical MgO powder obtained by decomposing Mg (OH)2 at 500˚C for three hours. The sample has a Langmuir surface area of about 187.69 m2∙g−1 in PVA 72000. The average particle size as deduced from the BET specific surface area is 12.22 nm. The value of the BET surface area and pore volume can improve the characteristics of MgO powder to make it suitable for catalytic activity and adsorption and dissociation of molecules, such as CH3OH, CH4, NO2, H2O, CO and CO2, toxic waste remediation, etc. [16] -[18] .
4. Conclusions
In summary, nanodispersed MgO particles with crystal size <15, 15 - 40 and >40 nm have been prepared by a wet chemical method at a low temperature. The results of the experiments show that surfactant and ligand play a key role in the composition of the precursor, which has determinative effects on the size of the obtained MgO nanoparticles.
The wet chemical method has been developed for the preparation of MgO nano with excellent basic catalytic activity. The preparation method of MgO nanoparticles is versatile and easily scalable, and is based on using cheap and abundant chemicals. The synthesis avoids extreme reaction conditions and high pressure treatment. The XRD results confirmed the hexagonal crystal structure of MgO. The FESEM clearly shows homogeneous nanostructures and remarkably different agglomerate sizes in different ligands and different surfactants, implying that the selection of the ligand and surfactant is a key factor for obtaining a high quality of nano-sized MgO via the wet chemical method.
Acknowledgements
This work was sponsored by the Research Institute of Petroleum Industry and the Department of Chemistry, University of Kharazmi, Tehran, Iran.
NOTES
*Corresponding author.