Two-Generation of Sphalerite from Pb-Zn Mineralization of Riasi Inlier, Jammu and Kashmir, India: Evidences from Sulphur Isotopes ()
1. Introduction
In the North Western Himalaya, lead and/or zinc sulphide mineralizations in form of pockets/lenses and veins have been reported long back from the Precambrian Sirban Limestone [1] . Roy et al., [2] suggested syngenetic stratigraphic control for these sulphides on the basis of absence of the intrusive body near the ore occurrences and field settings. Fairly strong but localized electromagnetic anomalies were suggested for the area indicating local enrichment of sulphide ore in the limestone body [3] . Recently new occurrences of sulphide mineralization are also observed from different formations present in the Riasi Inlier along the road and railway cuttings from areas located on the southern bank of the Anji Khad making the Inlier more interesting from mineralization point of view. Not much published work is available on the metalogenetic aspect of the Sirban Group except the work of Nayak and Sharma [4] , for the sulphide mineralization and Srivastava and Kumar [5] for the magnesite mineralization in Panthal area. In the present paper, stable sulphur isotope study of lead and zinc ores from all part of the Riasi Inlier is reported.
2. Geological Setting of the Area
In the NW Himalaya, sulphide mineralizations known to occur in the Riasi Inlier in Riasi District (J & K) which forms a thick Precambrian carbonate sequence. Riasi Inlier is the largest out of four inliers present in the area (Figure 1). Its length is 80 km and breadths vary between 8 to 20 km. On the basis of lithological similarities and unfossiliferous nature, the carbonates of Riasi inlier are correlated with limestone of Mt. Sirban of Hazara (Pakistan) and named as Sirban Limestone [6] . The Sirban Limestone is referred by different names as Trikuta Limestone, Jammu Limestone and Great Limestone in literature. Basic framework of geology of the area was first discussed by Medlicott [7] and since then the geology of the area has modified by number of workers [1] [8] . The whole carbonate sequence has been stratigraphically named as Sirban Group [9] . Based on the stromatolites and other micro-organism study, entire Proterozoic age was assigned for the Sirban Group [10] [11] . The Sirban Group has faulted and thrusted southern contacts with Murree Formation and Siwalik Group. It consists of a huge thick pile of dolomitic limestone which are the oldest rocks exposed in the area. It makes an unconformable contact with younger Tertiary rocks. Sirban Group is classified by different workers from time to time [12] [13] . Most recently the Sirban Group is classified into two distinct Formations [9] . The lower one which is essentially represented by calcareous sequence is named as Trikuta Formation. On the basis of lithology Trikuta
![]()
Figure 1. Geological map of Riasi Inlier between Thalwal and Chenab River (modified after R. S, Jamwal and B. D. Thappa, 1992). Inset map is showing regional geology of Jammu region (after Ganssar, 1964).
Limestone is divided into ten mapable units broadly comprising cherty and non-cherty dolomite, calc-argillite, calc-arenite, slate, flaggy limestone and stromatolitic limestone/dolomite. The upper one is named as Khairikot Formation which is mainly represented by areno-argillaceous rocks and it is divisible into two mapable units composed of chert-breccia, quartzite, slate and variegated shales with a single stromatolite bearing band of dolomite.
The regional structure in the area is represented by WNW-ENE trending doubly plunging anticline with rocks of Sirban Group in the core fringed by younger Tertiary rocks with marked angular unconformity. The Main Boundary Fault [14] is the major thrust of importance in the region which was termed as Jammu Fault and Vaishno Devi Thrust [15] . The general trend of rocks of Sirban Group in the area is WNW-ESE and the rocks are generally northerly dipping with some exceptions of southerly dip with an angle of 30˚ to 70˚. The pattern of prominent joints have NW-SE, N-S and NE-SW trend with steep to vertical dips. The dolostone is highly mineralized at places.
3. Pb-Zn Mineralization in the Area
The Pb-Zn mineralization in the area is present in form of pockets and lenses of galena and sphalerite with or without pyrite and are reported from Bakkal, Sersandu, Chanalakot, Paddar Nala, Khairikot, Rohotkot, Rad nala and Lower Darabi areas. Many old working in form of abandoned shafts and adits are scattered around some of these areas (Figure 2(a)). At places caprocks/gossans are also noticed (Figure 2(b)). The ore minerals present in the area are galena, sphalerite, pyrite and marcasite in order of their abundance and the gangue minerals associated with ores are calcite, quartz and dolomite. The mineralization is present with variable galena/sphalerite ratio. The galena dominated mineralization is mostly concentrated along the contact of dolostone and quartzite in the upper Khairikot Formation (Figure 2(c)). The lenses, veins, pockets and stringers of galena along with little sphalerite are found within the quartzites as well within with cherty dolostone. Locally veins of galena are also observed along the bedding and joint planes. While the sphalerite dominated mineralization is present as pocket within calcite veins intruding the dolostones and as veins in dolostones of the Lower Trikuta Formation. The Zn rich mineralization here occurs as the rich pockets of sphalerite with typical zoning of pyrite with less galena (Figure 2(d)).
The detailed petrographic studies under microscope reveal the presence of some other ore minerals as well. These minerals were identifies as marcasite, anglesite, gallite, sulphosalts as tennantite and lollingite and some other unidentified minerals apart from major galena, sphalerite and pyrite. Based on the field and ore microscopic studies the generalized paragenetic sequence for the Riasi Pb-Zn minralization is represented in Figure 3.
![]() (a) (b)
(a) (b)![]() (c) (d)
(c) (d)
Figure 2. (a) Old workings in the area; (b) The caprocks/gossans in Bakkal and Sersandu areas; (c) Galena mineralization in form of pockets and lenses along the contact of dolostone; (d) Zoning of pyrite in calcite.
![]()
Figure 3. Paragenetic sequence of lead zinc mineralization in Riasi area.
4. Sulphur Isotope Geochemistry
Isotope geochemistry has been used as a powerful tool in the deciphering the genesis of ore deposits [16] - [18] . The study of sulfur isotope abundances in nature is rewarding because of the ubiquity of the element and the variety of chemical forms in which it is found. Theoretical calculations and laboratory investigations of sulfur isotope fractionation in chemical, microbiological and mineralogical systems lead to an understanding of the isotope distribution patterns in nature and give information regarding the modes of formation and subsequent histories of many kinds of sulfur bearing materials [19] . Sulphide minerals formed in isotopic equilibrium with a hydrothermal fluid provide valuable insights into the condition of mineralization. Sulphur isotopic studies have been carried out on the representative sulphides from the Pb-Zn mineralization of Riasi. The results of the sulphur isotopes are discussed in following paragraphs.
5. Samples and Analytical Methods
Ten powdered samples of sulphides (five-sphalerite and five-galena) were analysed for sulphur isotopic study at Indiana University (USA). Galena and sphalerite samples hosted in different lihounits as well as from different areas were collected and utilized for the sulphur isotope geochemistry. Out of the ten samples analysed for the sulphur isotope, samples from both Khairikot Formation and Trikuta Formation were taken. In order to minimize the influence of other sulphides on each other, the sphalerite and galena were first handpicked and then crushed to 40 to 60 meshes. Sphalerite and galena were further separated using binocular microscope. Sulphur isotope compositions were obtained by using EA-IRMS (Elemental Analyser-Isotope Ratio Mass Spectrometer) at University of Indiana (USA). For determination of sulphur-34 the bulk material must first be converted to pure SO2 to permit analysis by IRMS. In this technique, samples are placed in clean tin capsules and loaded into an automatic sampler. They are then dropped into a combustion furnace held at 1080˚C where they are combusted in the presence of an excess of oxygen. The tin capsules flash combust causing the temperature in the vicinity of the sample to rise to ca. 1700˚C. The gaseous products of combustion are then swept in a helium stream through tungstic oxide and zirconium oxide combustion catalysts and then reduced over high purity copper wires. Water is removed by a Nafion™ membrane, permeable to only water. Sulphur dioxide is separated by a packed column gas chromatograph held at an isothermal temperature. The resultant SO2 chromatographic peak enters the ion source of the IRMS where it is ionised and accelerated. Gas species of different mass are separated in a magnetic field and simultaneously measured by a Faraday cup universal collector array. For SO2, masses 64, 65 and 66 are monitored.
6. Result and Discussion
Sulphur isotope compositions of sulphide minerals (sphalerite and galena) from Pb-Zn mineralization of Riasi are listed in Table 1. The overall sulphur (δ34S) values of sphalerite is ranging from +9.67‰ to +34.42‰. δ34S values of sphalerite within the calcite veins intruding Trikuta Formation and hosted by the dolostones are varying between +9.67‰ to +10.59‰ with an average of +10.13‰. The sphalerite present with galena and hosted by cherty dolostone and quartzite of Khairikot Formation, however show δ34S values varying between +18.39‰ to +34.42 ‰ with an average of +26.21‰. The galena associated with the sphalerite, however show a lower δ34S values ranging within limit of +19.41‰ to +23.89‰.
The Riasi limestone belongs to the second sedimentary cycle of Himalaya which is normally characterized by the penecontemporaneous volcanism in the lower most part of sedimentary cycle [20] . The basement for Sirban Group, however, is not exposed in the area. The plateformal carbonate sequence of Sirban Group is characterized by the presence of stromatolites and other microbiota [11] [21] . Several carbonate facies in the Sirban Group have been identified which show cyclic repetition [22] [23] . This indicated that the entire sequence was deposited in 3 to 4 transgression-regression cycles and the depositional environment shifted supratidal to intertidal and subtidal conditions [23] .
The mineralization in the Riasi Inlier is seems to be controlled by lithology on the regional basis. It has been observed that galena occurs in cherty dolostone and over lying quartzites whereas sphalerite is found only in dolostone. Bracciated dolomites are commonly observed in the area. Brecciation is an important process of ground preparation for the localization of most carbonate-hosted mineral deposits. It provides excellent conduits and receptacles for the introduction and movement of hydrothermal fluids and precipitation of the ore minerals. The process of brecciation may be due to the dissolution and transport of Ca carbonate by meteoric water in terranes predominantly underlain by carbonate rocks.
The sulphure isotope values for the sphalerite shows a wide range from +9.67‰ to +34.42‰ with two different peaks. The differences in δ34S values in sphaletrite of two groups may be interpreted to be formed by two different ore fluids of different isotopic compositions.
![]()
Table 1. Sulphur isotope values of Sphalerite and Galena from Riasi.
7. Conclusion
Based on the sulphur isotopic data, it is concluded that the sphalerite ores of the Pb-Zn mineralization in Riasi are of two-generation; one with lower isotopic values varies from +9.67‰ to +10.59% and the other with higher isotopic ratios varies from +18.34‰ to +34.42‰. The two-generation of sphalerite theory is also confirmed in the ore petrographic studies and also in the fluid inclusion studies. Sphalerite(I) has lower sulphur isotopic values and is mostly confined Lower Darabi area of Trikuta Formation. Whereas Sphalerite(II) has a higher δ34S values and is present all over with the galena. Involvement of an isotopically lighter sulphur source is suggested to explain the calculated δ34S values of Sphalerite(I) of the Darabi area [24] . The biogenic nature of much of the dolostone in the area suggests high contents of organic matter, rich in isotopically light carbon and sulphur. It is likely that, as a result of reaction with the host rocks, the mineralizing solutions become enriched in isotopically light sulphur derived from organic matter and diagenetic pyrite in the dolostone [24] [25] . During the deposition cycle of this carbonate sequence, supratidal environment has also been reported with deposition of anhydrite. The higher positive values of δ34S suggest that reduced sulphur for the sulphide mineralization is derived from evaporites by thermo-chemical sulphate reduction, whereas 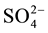 is directly originated from evaporites [26] . The main reason for the wide ranges in δ34S in sulphides in two areas may be explained by mixing between two sulphur bearing fluids with different isotope compositions.
is directly originated from evaporites [26] . The main reason for the wide ranges in δ34S in sulphides in two areas may be explained by mixing between two sulphur bearing fluids with different isotope compositions.
Acknowledgements
We are very grateful to Mr. Benjamin. S. Underwood of the Stable Isotope Research Facility Indiana University (USA), for carrying out stable isotope analysis. The financial support in form of a research project to PKS from University Grant Commission and fellowship to ID under DST-Inspire Fellowship scheme of Department of Science and Technology is gratefully acknowledged.