Thermal and Photochemical Effects on the Fluorescence Properties of Type I Calf Skin Collagen Solutions at Physiological pH ()
1. Introduction
It is well known that mammalian collagens exhibit weak intrinsic UV fluorescence. Observed fluorescence spectra depend on the age and previous history of the sample. In nascent collagen, the only fluorescent chromophore is tyrosine (peak absorption/emission maxima at 275/305 nm). However, post-translational ground- and/or excited-state modifications can result in additional fluorescent products derived from tyrosine oxidation that absorb and emit at longer wavelengths (e.g. DOPA (285/315 nm) [1] , dityrosine (325/400 nm) [2] , DOPA-oxida- tion products (emitting from 330 - 470 nm) [3] and advanced glycation end products, involving a reducible sugar and lysine (370/450 nm) (AGE) [4] - [8] ). We do not expect to find AGEs in these experiments, because there are no sugars in our reaction mixtures. We have found that oxidative changes can be observed even under storage in the solid state at 4˚C in the dark.
These compounds are photolabile, the predominant photo-product being photo-dimerization to dityrosine [9] [10] . Photo-induced dityrosine formation and fluorescence disappearance (“fading”) of DOPA oxidation products (“oxidation products”) attending exposure to UV radiation have been used as convenient markers of the extent of photolysis [11] [12] . This photolability suggests the possibility of collagen or its oxidation products acting as in situ photosensitizers and/or phototoxic agent.
These ground- and excited-state processes, and the relationships between them are not well described, and a better understanding of them is necessary. We are therefore systematically investigating the time- and UV-de- pendent fluorescence properties of type I calf skin collagen solutions buffered at pH = 7.4. Our initial results are reported in this communication.
2. Methods
2.1. Collagens
Three commercial purified collagen samples from the same manufacturer were used; newly-purchased calf-skin collagens (#092014), a “new” sample purchased in 2012 (#072012) and an older sample purchased in 2005 (#072005) that had been stored in the solid state in the dark at 4˚C for ~7 years before analysis in 2012. Samples #072012 and sample #072014 were analyzed in June-July 2012 and September 2014 respectively as soon as they were received, and their spectral and kinetic properties were virtually identical. Sample #072005 was analyzed at the same time as #072012 (June-July 2012).
2.2. Fluorescence Spectroscopy
Fluorescence emission spectra of collagen samples were recorded on a Perkin-Elmer 650 - 40 fluorescence spectrometer, equipped with a thermostatted sample compartment, in conjunction with a circulating bath (Lauda, K-2R; Brinkmann Instruments, Westbury, NY, USA). Horizontal entrance and exit slits in the sample compartment allow measurement of as little as 0.3 ml, and optimize measurement of turbid solutions. Optical quartz 1.0 cm cells (Hellma Cells, Inc., Plainview, NY, USA) were used to collect fluorescence emitted at right angles to the excitation beam. Because of the weak fluorescence, the band widths of both excitation and emission monochromators were set at 5 nm. Fluorescence spectra were recorded manually at excitation wavelength 270 nm. Possible photochemical activity was limited by filling the cuvettes to capacity (3.0 ml), and minimizing exposure to excitation light. The time constant was 2.0 s. All spectra were corrected for instrumental distortions.
Photochemical changes occurring in these solutions were studied as follows: one (1.0) ml of 0.05% collagen solutions were irradiated in a thermostatted cuvette (Hellma Cells, Plainview, NY, 11803, USA). Readings were taken at excitation/emission wavelengths of 270/300 (tyrosine), 270/330 (DOPA), 270/360 (“interacting” tyrosine or DOPA oxidation product(s)), 270/400 (link between excitation at 270 nm and 325 nm), 325/400 (dityrosine), and 370/450 nm (AGE’s) (not relevant here); DOPA oxidation product(s) [12] [13] . The choice of excitation/emission wavelength pairs was chosen to limit interference from overlapping bands. Irradiations were carried out with a 4 W UVG-11 hand lamp (emitting primarily 254 nm superimposed on a UV continuum; (output 6.6 W/m2)). The geometry was such that the irradiation impinged on the entire sample.
The resulting fluorescence intensity, If(t) vs. irradiation time t plots were analyzed kinetically. The 270/360 nm fluorescence fading was analyzed as a second-order plot of 1/I(t) vs. t to afford a second order rate parameter [11] r2, whose slope is proportional to a molecular rate constant for disappearance of the (unknown) fluorophore at 270/360 nm, is:
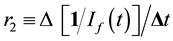 (1)
(1)
The build-up of 325/400 nm fluorescence attending photolysis (dityrosine formation) was plotted as I(t) vs. t to give a quasi-linear plot [9] whose slope affords a first order rate parameter, proportional to a molecular rate constant for build-up of the dityrosine fluorophore at 325/400 nm, is:
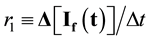 (2)
(2)
Both r1 and r2 are proportional to fluorophore concentration [9] [11] . Changes in the other fluorescence pairs were kinetically complicated alterations and were not amenable to simple analysis (see [12] for results with Skh-1 hairless mouse collagen).
Although the dimensions of r1 and r2 are different from each other, the ratio of slopes r2/r1 serves as a convenient empirical tool for comparing the individual collagen samples.
2.3. Statistics
Data were analyzed by the Student’s t test as Mean ± S.D. of the slopes of fluorescence fading/build-up curves from 3 separate experiments.
3. Results
The only fluorescent chromophore in nascent collagen is tyrosine. However, post-translational modifications in amino acid structure result in additional fluorescent oxidation products, so that collagen fluorescence spectra depend on the age and previous history of the sample. This result is illustrated in Figure 1. The fluorescence spectra of new samples #072012 and #092014 (not shown) are very similar to each other, showing tyrosine fluorescence at 300 - 305 nm as the predominant peak. However comparison of these spectra with that of pure tyrosine [14] shows a slight broadening of the overall fluorescence envelope, due to small amounts of DOPA and its oxidation products (Figure 2). The older sample, #072005, shows significant diminution of the tyrosine peak at 305 nm (I(072005)/I(072012) = 0.57) as well as the more obvious appearance of additional longer wavelength bands that can be ascribed to a mixture of DOPA, DOPA oxidation products and dityrosine [11] - [13] .
UVC (254 nm) irradiation of collagen causes a rapid decrease of the 270/360 nm fluorescence (“photolabileoxidation product”) and concomitant increase in the 325/400 nm band (dityrosine formation). Other fluorescence
![]()
Figure 1. Corrected fluorescence emission spectra of calf skin type I collagen samples 0.05% in 0.1 M phosphate buffer, pH = 7.4, T = 24˚C - 26˚C, Excitation at 270 nm. Solid line: fresh sample #072012, Dotted line: old sample #072005 that had been stored in the dark for ~7 years prior to analysis. Spectra are normalized to the 305 nm peak of #072012.
![]()
Figure 2. Corrected normalized fluorescence spectra of purified tyrosine [14] (solid line) and of sample #072012 shown in Figure 1 above (dotted line). Oxidation products in #072012 are revealed by spectra broadening at long- wavelength edge.
pairs are kinetically complicated alterations and not amenable to simple analysis. Similar results were previously obtained with Skh-1 hairless mouse collagen [12] [13] .
The 270/360 nm band shows a linear relationship between reciprocal fluorescence intensity, 1/If (t) vs. time, t. Figure 3(a) compares fading results of (270)360 nm fluorescence from lot #072005 and #072012. The value of r2/r1 = 2.31 ± 0.40. Covalent dityrosine (325/400 nm fluorescence) is formed from a metastable steady-state tyrosyl radical, where the tyrosyl steady state concentration is proportional to the amount of tyrosine [9] . In such cases, plots of If(t) vs. t are quasi-linear, [9] ; (Figure 3(b)), with r2/r1 = 0.246 ± 0.097 (see Table 1 for results).
Table 1 clearly shows the reciprocal relationship between the rates of decrease in the 270/360 nm fluorescence (oxidation products) and concomitant increase in 325/400 nm fluorescence (dityrosine) where the relative rates depend on the age of the collagen sample. Thus, r2 > r1 in sample #072005, whereas r2 < r1 in #072012. It therefore appears that the oxidation products are formed at the expense of tyrosine.
4. Discussion
We have investigated the thermal and photochemical stability of type I calf skin collagen at physiological pH. Preparations lot #072012 and #092014 had fluorescence spectra that were virtually identical, and were similar to the spectrum of pure tyrosine. Although both collagen samples were analyzed on arrival, there was nonetheless a small trace of DOPA oxidation products in them that were lacking in pure tyrosine (Figure 2). Since these collagen samples were purified from calf skin, it is quite possible that some oxidation had occurred in vivo. Lot #072005 had been stored at 4˚C in the dark and there was evidently a significant amount of oxidation at the expense of tyrosine. Since these additional oxidation products are photolabile, their overall effect is to decrease the stability of collagen with time.
Dityrosine formation by UV can be analyzed as a pseudo-first-order steady-state reaction [9] , where [Tyr]SS is directly proportional to the initial concentration of tyrosine in the collagen sample.
The second order fading of the (270)360 nm species suggests a “double molecule”, which we have previously attributed to an “excimer-like” interaction between two tyrosine molecules in close proximity [12] . Alternatively, it may be a stable, as yet uncharacterized, DOPA oxidation product. One might expect such an excimer-like interaction to favor dityrosine formation. In fact, the observed reciprocal relationship between the 325/400 nm and the 270/360 nm species suggests that the latter is formed at the expense of dityrosine. This finding argues against an interaction involving two proximal tyrosine molecules, but is consistent with the formation of a stable oxidized fluorescent product.
![]()
![]() (a) (b)
(a) (b)
Figure 3. (a) Second-order fading of 270/360 nm fluorescence (oxidized product(s)) as reciprocal plot vs. time for sample #072005 (black dots) and #072012 (white dots). This species fades significantly faster in the older sample #072005; (b) First-order build-up of 325/400 nm fluorescence (dityrosine) as fluorescence vs. time for sample #072005 (black dots) and #072012 (white dots). This species accumulates significantly slower in the older sample #072005.
![]()
Table 1. Normalized fluorescence fading of the (270)360 nm band as 1n/If (t) vs. t and normalized fluorescence build-up of (325)/400 nm as If(t) vs. t in calf-skin collagen sample #072012 (fresh sample) and #072005 (~7 years old) in 0.1 M PO4 buffer, pH = 7.4 (see text). Parameters r1 and r2 are defined in the text. The ratio r2/r1 is an empirical measurement proportional to the relative amounts of oxidation product(s)/dityrosine.
*Mean ± S.D. of three experiments.
Both photochemical photo-dimerization [9] [10] and ground state reactions [3] of tyrosine involve formation of a tyrosyl radical intermediate, which decays either by forming covalent dityrosine, or by reaction with O2 and consequent formation of DOPA, and higher oxidation products [3] . In the absence of UV radiation, the relative abundance of oxygen favors the latter route, but a small amount of dityrosine was also formed in the dark in sample #072005.
Tyrosyl radical formation usually requires a highly oxidative agent because of the high redox potential of the tyrosyl/ tyrosine couple. The photochemical formation of dityrosine uses the absorbed photonic energy to accomplish tyrosyl radical formation. Ground state oxidation of tyrosine residues in collagen is most likely to involve water of hydration [15] . Our collagen samples, no doubt, were hydrated even in the solid state. The electrical properties of hydrated collagen have been studied by several workers (for quick review, see [15] and references therein). Tomaselli et al. [15] found that the adsorption of water onto solid bovine Achille’s tendon (BAT) caused a dramatic increase on the values of dc conductivity σ that is both temperature- and hydration parameter (h)-dependent (as much as 8 orders of magnitude at h = 0.24). Increasing hydration decreases the activation energy, and moderate hydration levels, confer semiconductor-like properties on the collagen, with water possibly acting as an impurity [15] . Although the exact mechanism of tyrosyl formation is unclear, we can speculate that moderate hydration lowers the activation energy sufficiently to allow tyrosine oxidation via electron transfer (ET), proton transfer (PT), or proton-coupled electron transfer (PCET) (for review of PCET, see [16] ). In buffered solution, tyrosyl formation could also have been facilitated by the presence of phosphate buffer [17] .
5. Conclusion
Collagen fluorescence spectra and reaction kinetics depend on the age and previous history of the sample. Autoxidation can take place even in the solid state at 4˚C in the dark. There is a reciprocal relationship between the rates of decrease in the 270/360 nm fluorescence and concomitant increase in 325/400 nm fluorescence, resulting in a “Ying-Yang” relationship between r1 and r2. This relationship results because both ground state autoxidation and excited state photo-dimerization proceed via a common tyrosyl radical intermediate. Water of hydration appears to play a role in generating tyrosyl radical, particularly in dark autoxidation reactions.
Acknowledgements
This work was supported in part by DOD Grant #911 NF-10-1, MBRS Grant #GM08248, and RCMI Grant #. RR 3034. There are no conflicts of interest.
Abbreviations and Acronyms
Fluorescence excitation and emission wavelengths (nm): e.g. excitation at 270, emission at 360 nm = (270)/ 360 nm; DOPA: 3,4-dihydroxyphenylalanine; AGE: advanced glycation end products; r2 ≡ rate of 1/(270) 360 nm fluorescence disappearance; r1 ≡ rate of (325)/400 nm fluorescence build-up; BAT: Bovine Achille’s Tendon; ET: Electron Transfer; PT: Proton Transfer; PCET: Proton-Coupled Electron Transfer.