1. Introduction
The useful properties of soft ferrites in low and high-frequency equipment and their roles in microwave devices, power transformers, rod antennas, read/write heads for high speed digital tapes have attracted much interest for researchers [1] . Soft ferrites are divided into two major categories; the manganese-zinc (MnZn) ferrite and the nickel-zinc (NiZn) ferrite. However, in the recent, CoZn ferrite has blossom in researches involving high frequency devices. Soft ferrites can be used in the areas of low and high frequency devices, microwave devices, power transformers, read/write heads, antennas for communication and for interference suppression and absorption. The advantages of using soft ferrites are listed as follows:
Small eddy current loss at high frequency, chemical stability, high resistivity, low dielectric losses and high permeability, wide range of operating frequencies, low cost, light weight, large material selection, high Curie temperature.
The electromagnetic properties of ferrites materials are highly dependent on a number of factors. These factors include preparation techniques, temperature, and purity of materials used in sample preparation. In a number of researches under taken, polycrystalline sample BiFeO3 synthesized at low temperature showed that the crystallite size increases and strain in the crystallite decreases with increasing sintering temperature thereby decreasing the lattice parameter [2] .
Polycrystalline NiFe2O4 was prepared by solid state reaction from nano size powder of NiO and Fe2O3 which were synthesized by wet chemical method [3] . This method produced an inverse spinel single phase which was confirmed by the X-ray diffraction patterns. SEM micrographs of the prepared samples showed that the grain size increases with increasing sintering temperature.
In [4] , they prepared nano zinc niobate powder using high energy ball milling; the powders produced were further sintered at different temperatures. Samples prepared at temperatures ranges of 975˚C to 1100˚C, exhibited a single columbite phase with a relative densities range of 87% to 99%.
Ferrites are manufactured by processing a composition of iron oxide mixed with other major constituents such as oxides or carbonates of either manganese and zinc, nickel and zinc, cobalt and zinc. The basic process is common to most ceramic process technologies and can be divided into four major functions: powder synthesis, pelletization, sintering and finishing [5] [6] . This study intends to explore the impact of the sintering temperature on the microstructure of the Co-Zn ferrite since the electromagnetic properties are very sensitive to their micro-structure.
Among the current methods for synthesis of cobalt-zinc ferrite, the mechanical alloy technique stands as a good candidate for large scale production nano ferrites. The mechanical alloy method is quite easy, fast, and inexpensive, and above all friendly to the environment.
In this study, CoO + ZnO + Fe2O3 were mixed together and milled for 12 hours using high energy milling machine before they are sintered for 10 hours with different sintering temperature. The sintered samples are then used to study the changes on its structural properties.
2. Method
Co0.5Zn0.5Fe2O4 was prepared by mechanical alloying technique using the normal combination of iron oxide and other major constituents (Co-Zn). Materials used for the production of nano ferrite in this study are Fe2O3 (Alfa Aesar) (99.95%), CoO (Alfa Aesar) (99.99%) and ZnO (Alfa Aesar) (99.99%). High energy milling was carried out in a SPEX 8000D shaker for 12 hours. The ball to powder mass ratio used was 10:1. The milled powder was then prepared into rectangular pellets of 3 mm, 5 mm and 7 mm thick, which was further used for dielectric characterization not mentioned in this paper. The powder and pellets were then sintered at temperatures of 500˚C, 700˚C, 900˚C and 1100˚C for 10 hours each respectively.
X-ray diffraction (Phillips Expert MPD System) coupled with graphite monochromator, operating at 40 kV and 30 mA using CuKα radiation (λ = 0.1542 nm) at a scan rate of 0.332 θ/sec was used to analyse all samples used in this study. The different samples sintered at different temperatures were analysed with Scanning Electron Microscope (SEM), Transmission electron microscope (TEM), and Energy Dispersive X ray (EDX).
3. Results and Discussion
X-ray patterns of the synthesized samples of Co0.5Zn0.5Fe2O4 as burnt powders and sintered at different temperatures are shown in Figure 1. Observation on the XRD result for sintered sample at 500˚C showed that Co-Zn ferrite existed as a major phase and ZnO as a minor phase. Analysis shows that the zinc ions do not react in the formation of the ferrite. No additional phase was detected as the sintering temperature begins to increase
![]()
Figure 1. XRD spectra of Co-Zn-ferrites powder prepared by mechanical alloying at different sintering temperatures.
and single phase spinel structure resulted as a result of the sintering [7] . Observations on the XRD pattern for the 900˚C and 1100˚C showed strains (Figure 1) which might be attributed to remnant stress through chemical reaction process [8] [9] . The small crystallite sizes of samples are evident from the broad peaks patterns as shown in Figure 1 [10] .
The interpretation of the XRD spectra for all sintering temperatures was achieved using the data base of the Joint Committee on Powder Diffraction Standards [11] . Scherer equation was employed in the calculation of the crystallite size of powders under study shown in Equation (1) [12] .
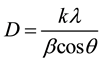 (1)
(1)
where D is the crystallite size, k is shape factor, λ is X-ray wavelength, β is full-wave half maximum in radians.
Analysis showed that the average grain size increased with increasing sintering temperature. In [13] , they stated that Zn promotes sintering and hence increases the grain size as the temperature increases.
As expected, between the temperature ranges of 700˚C to 1100˚C complete phase of Co0.5Zn0.5Fe2O4 begins to emanate with increasing peaks as the sintering temperature increases. Careful observation on Figure 1, showed that at 1100˚C, some strange peaks begin to appear. These new peaks at the (533) and (622) might be attributed to transition from the single spinel phase to the magnetisation phase and the loss of Zn2+ at higher sintering temperature. Zn2+ loss due to high temperature will result in stoichiometry imbalance which causes the Fe3+ ions to be converted to Fe2+ ions which in turn will lead to electrical charge balance of material composition [14] .
Increasing sintering temperature influences the increase in lattice parameter of samples which in turn causes the diffusion of zinc ions in to the tetrahedral sites of the samples. This assertion is supported by research undertaken by [15] .
As the sintering temperature increases for the milled powder, the intensity of all the samples also increases as evident in Table 1. Table is the summary the intensity as sintering temperature increases.
The lattice constant is calculated from the combination of the Bragg’s equation and d-spacing expression for cubic system (Equation (2)) [12] .
 (2)
(2)
where x is the lattice constant, and h, k, l are the peak numbers, λ is X ray wave number.
The lattice constants was calculated using Equation (2). The magnitudes of lattice parameter for the different sintering temperatures were obtained as 8.389 Å, 8.405 Å, 8.386 Å, 8.403 Å, and 8.361 Å for the 0˚C, 500˚C, 700˚C, 900˚C, and 1100˚C temperatures respectively. The irregular behaviour of the lattice parameter might be due to the initial change in evolution in grain growth and crystallization [16] [17] . The diffusion of zinc ions into the tetrahedral sites are the main causes of the change in lattice parameter of samples, due to sintering.
The X ray density (Dx) of samples were calculated using the given equation in (3), [12]
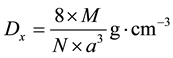 (3)
(3)
where M is the molecular mass of Co0.5Zn0.5Fe2O4 (g/mol), N = Avogadro’s number (6.022 × 1023 entities/mol), and “a” is the lattice constant (cm).
Calculated result presented in Table 2 showed that the density is in close agreement with published data (JCPDS, 1971 and JCPDS, 1947). As temperature increases, the density of the sample also increases for both the calculated and measured densities as shown in Figure 2.
The crystallite size as a function of sintering temperature is presented in Table 3 and interpreted in Figure 3. The crystallite size of the sintered as powdered was calculated using the Scherer relationship from the XRD lines. The full wave half maximum, (FWHM) value was converted to radian before computation in the Scherer equation. The conversion from degree to radian for the FWHM value was done by using the formula [12] ;
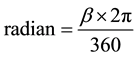 (4)
(4)
where β is the FWHM value in degree, π is 3.142.
The crystallite size calculated for the Co0.5Zn0.5Fe2O4 powder sample sintered at different temperatures of 500˚C, 700˚C, 900˚C, and 1100˚C produced values in the range of 21.43 - 63.38 nm. Result showed that the milled sample (without sintering) has already showed a distinct diameter from the rest of the sintered diameter.
![]()
Table 1. Intensity and 2Theta for samples sintered at different temperatures.
![]()
Table 2. Density of samples at different sintering temperature.
![]()
Table 3. Crystallite size of Co0.5Zn0.5Fe2O4 sample sintered at different temperatures.
![]()
Figure 2. Variation in measured and calculated density of MUT.
![]()
Figure 3. Crystallites of sample vs sintering temperature.
Growth of particles is observed from 500˚C to 1100˚C, this is due to the mechanical alloy method used in the preparation of the sample. As the temperature increases, a broader particle size begins to emanate due to the thermal energy supplied at this stage. Grain growth development started at above the 700˚C, where necking process starts to appear. The grain distribution at 1100˚C is wider than that of the 700˚C which is attributed to grain growth to larger sizes thereby creating more development of domain walls.
Scanning electron microscope (SEM) micrographs of Co0.5Zn0.5Fe2O4 particles sintered at different temperatures for 10 hours in air synthesised via mechanical alloy method is presented in Figure 4. The result from SEM analysis showed that the surface microstructure of sintered Co0.5Zn0.5Fe2O4 are bundle together with increasing sintering temperature from 500˚C to 1100˚C. It was also observed that the average grain size increased with increasing sintering temperature.
Energy dispersive X-ray (EDX) analysis was performed to confirm quantitative composition of elements in the Co0.5Zn0.5Fe2O4 nano particles after sintering at different temperatures. Result from the analysis as shown in Table 4 confirmed that the stoichiometric composition of mixture before sintering was also the same after sintering.
TEM micrograph shown in Figure 5 is of the ferrite powder obtained from the milling process and sintered at different temperatures. Samples to be studied were sonificated in acetone and dispersed uniformly on the TEM copper grid. The TEM micrograph revealed difference in size distribution as temperature changes. The particle size distribution obtained from TEM micrographs from Figure 5 showed that the average particles sizes are 18.0 nm, 20 nm, 39.5 nm, 48.5 nm, and 57.5 nm for the unsintered, 500˚C, 700˚C, 900˚C, and 1100˚C respectively.
4. Conclusion
Co-Zn ferrite nano powders were synthesized using the mechanical alloying method with a molar ratio of 1:0.5:0.5. The XRD spectra indicate the diffusion of ZnO into the tetrahedral sites followed by CoO into the octahedral sites. The diffusion occurred during the early stage of the milling process. The crystal size calculated exhibits an increase in nano dimension due to the sintering process with a corresponding increase in density of
![]()
Figure 4. SEM micrograph of Co0.5Zn0.5Fe2O4 nano particles sintered at (A) 500˚C (B) 700˚C (C) 900˚C and (D) 1100˚C.
![]()
Table 4. Elemental composition of the Co0.5Zn0.5Fe2O4 sintered powder at 500˚C.
![]()
![]()
Figure 5. TEM micrograph of Co0.5Zn0.5Fe2O4 nano particles sintered at (a) 500˚C (b) 700˚C (c) 900˚C and (D) 1100˚C.
the samples. This behaviour is also evident from the SEM analysis showing increase in compactness of the samples as temperature increases. The micrograph of SEM showed that the synthesized powders began to agglomerate as the sintering temperature increases. TEM micrograph showed that the powders are of nanosize dimension and the values of the sizes increase with increasing sintering temperature.
Acknowledgements
The researchers wish to thank the Universiti Putra Malaysia for the fund provided under grant Vot no. 5526200.