Surface Modification of Commercial Activated Carbon (CAG) for the Adsorption of Benzene and Toluene ()
1. Introduction
In recent years, with increasing population and industrial activity, intensified concerns regarding water quality, among these groundwater has received special attention. Contamination of water and soil by volatile organic compounds (VOCs), as a result of accidents or spills (fuel) and wastewater industrial today poses serious problems to public health [1] . Volatile organic compounds such as benzene and toluene are found frequently in the wastewater from paint industries, adhesives, plastics, petrochemicals, among others [2] . Benzene and toluene are flammable, toxic and carcinogenic substances, their presence in aqueous environments have a negative impact on water quality and thus endanger the welfare and public health [3] -[5] .
Activated carbon (CA) has been used as adsorbent for the removal of a wide variety of organic and inorganic pollutants from aqueous media [6] . This adsorbent is widely used in the treatment of waste water due to their high surface area between 500 - 1500 m2/g, well-developed internal structure of micropores, as well as a large variety of surface functional groups [7] . Exceptional adsorption of organic compounds by activated carbon is attributed to their hydrophobicity and ability to adsorb molecules with molecular weight between 45 - 130 g/g mol [8] . CA adsorption has been cited by the North American Environmental Protection Agency (USEPA) as one of the best available technologies in environmental control [9] .
The liquid phase adsorption is generally a more complex phenomenon than the adsorption of gas phase. The liquid phase adsorption is influenced by various factors such as solubility of the adsorbate, solution pH, temperature and chemistry of the adsorbent surface [10] . The adsorption of organic compounds in aqueous phase by CA mechanism involves two main types of interactions: electrostatic and dispersive [11] .
Although the performance of activated carbon as adsorbent for a wide variety of organic compounds is known [12] , numerous studies are developed to study surface modifications of CA’s, which could help to improve these materials, such as specific adsorbents of organic and inorganic contaminants present in wastewater [13] - [15] .
The present study reports the influence of surface modification of HNO3 treated CAG for adsorption of benzene and toluene from aqueous solutions, and use of the inferential statistical analysis for the treatment of experimental adsorption data. The adsorption of benzene and toluene by CA can be a viable alternative for the removal of these compounds from aqueous systems, thus minimizing contamination. Since the aquifers are water supply sources for human consumer.
2. Materials and Methods
2.1. Adsorbents
Commercial granular activated carbon (GAC) of Synth®mark, particle size: 1 - 2 mm was used as an adsorbent (where was GAC source from). The adsorbent was washed and boiled for three hours in deionized water. It was subsequently dried in an oven (105˚C) for 24 h to make sample (CA-1).
Adsorbent CA-2 was obtained from CA-1 by treated with 6 M HNO3, according to the procedure described by Elhendawy [16] . Briefly 100 mL of 6 MHNO3 were used to treat/10g CA-1 under reflux at 100˚C for 2 h. The adsorbent was subsequently washed with deionized water to neutral pH and dried at 105˚C for 24 h to make sample (CA-2).
2.2. Characterization of the Adsorbent
2.2.1. Specific Surface Area and Total Pore Volume (Vp)
The values of specific surface area and total pore volume of the adsorbents were determined from N2 adsorption isotherms at 77 K, using a porosimeter MICROMERITICS TRISTAR II.
2.2.2. Determination of pH
The pH of activated carbon was determined according to previously reported standard method ASTM D3838- 05 [17] .
2.2.3. Characterization of the Adsorbent Surface by FTIR
Analyses were performed on spectrophotometer Fourier transform infrared (FTIR) Thermo Scientific Nicolet (IS 10 model) [18] .
2.2.4. Characterization of Functional Groups on the Surface of the Adsorbent
The characterization of the surface functional groups of (CAG) was performed according to the Boehm methodology [19] [20] . In the determination of acid groups 5.0 g of the adsorbent were suspended in 50 ml of 0.1 M standard solutions (sodium hydroxide, potassium bicarbonate, KHCO3 and sodium carbonate, Na2CO3). The vials with the adsorbent suspensions were closed and shaken in a thermostatic bath (27˚C) at 140 rpm for 24 h. The suspensions were filtered (0.45 μm Millipore) and titrated with a solution of hydrochloric acid HCl (0.1 M).
In the determination of basic groups using 2.0 g of CAG were suspended in 20 ml of 0.1 M-hydrochloric acid, under same experimental conditions as described for the determination of acid groups. After equilibration, the suspensions were filtered (0.45 μm Millipore) and a 10 ml aliquot was titrated under continuous stirring (magnetic stirrer) against 0.1 M. sodium hydroxide. The identified functional groups CAG were calculated as mmol/g (MAS).
2.3. Tests for Adsorption of Benzene and Toluene
2.3.1. Solutions of Organic Compounds Used for Adsorption
Organic solutions (60 mg/L) were prepared from benzene (99.5% purity) and toluene (99.8% purity) according to the studies done by Lu, Su and Hu [21] .
2.3.2. Analytical Determination of Organic Compounds
The extraction methodology used to analyze benzene and toluene in the water samples was automated headspace method which is based on 6040D Supelco/Sigma-Aldrich [22] . Determination of benzene and toluene in water samples was performed according to EPA 0010 method [23] . Aliquots (15 ml) of the samples were placed in 20 ml vials and sealed with aluminum seals, and teflon septum. The analytes were quantified by a gas chromatograph (Varian, CP 3800) connected to a mass spectrometer (MS-320 CTC Analytics) with auto-sampled system and headspace and flame ionization detector (GC-FID). The chromatograms were obtained and processed by Varian Star Workstation software.
2.3.3. Kinetics and Balance Adsorption of Benzene and Toluene
Kinetic assays and adsorption equilibrium were performed in thermostatic bath (25˚C) at 140 rpm for contact time of up to 25 minutes. In the assays used aqueous solutions, concentrations of up to 60 mg/L. The adsorption kinetics tests were performed at 3, 5, 7, 9, 13, 15, 17, 20, 22 and 25 minutes. Each assay consisted of about 1.0 g of adsorbent for every 100 ml organic solution placed in closed glass vials and immersed in a thermostatic bath. Kinetic mathematical model of pseudo―second order in the linear form was used to correlate the experimental data obtained using Equation (1).
The adsorption isotherms were obtained by suspending about 1.0g adsorbent in 100 mL of different organic solution concentrations (1.0, 1.5, 3.0, 5.0, 10.0, 15.0, 20.0, 25.0, 30.0, 40.0, 50.0 and 60.0 mg/L), contained in closed glass vials (mouth grinded) and immersed in thermostatic bath for 25 minutes. Equation (2) shows the mathematical model of Langmuir, which was used to correlate the experimental data. Equation (3) was used to calculate the amount of adsorbed benzene and toluene (Qe mg/g). The percent removal of compounds by adsorption was calculated according to Equation (4).
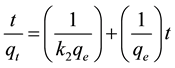 (1)
(1)
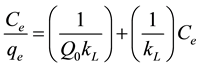 (2)
(2)
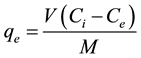 (3)
(3)
 (4)
(4)
where: qe and qt is the amount of benzene or toluene adsorbed (mg/g) at equilibrium and at time t (min), respectively; k2 is the rate constant pseudo second order (g/mg-min); Ci and Ce are benzene concentrations(mg/L) at initial and equilibrium respectively; Qo, maximum adsorbed amount (mg/g); kL Langmuir constant (L/mg); V is the volume of solution (L); M is the mass the adsorbent (g) and R is the adsorption efficiency of benzene or toluene (%).
2.3.4. Tests for the Adsorption of Benzene and Toluene Fixed Bed
A bird with the following dimensions was employed: a glass column of 40 cm in height; bed height (HL) of 24.2 cm, bed internal diameter (ID) of 2.5 cm, adsorbent mass (m) of 63.0 g. The conical base and the top of the column was filled with glass beads (0.5 mm diameter). The following values for the volumetric feed flow rate of the column were used: 70 mL∙min?1 (Q1) and 100 ml∙min?1 (Q2) in downward flow.
Both adsorbents (CA-1 and CA-2) were used separately in benzene and toluene and two values of volumetric flow rates (Q1 and Q2 respectively) were obtained. A total of eight runs were performed using 12 L of a 60 mg/L organic solution per run and contact time of 172.5 minutes and 120 minutes were used respectively for the values of volumetric flow rate of Q1 and Q2 at ambient temperature (25˚C). During the adsorption process were collected 19 samples were collected from which were used to determined the residual concentrations of benzene and toluene by gas chromatography headspace extraction.
2.3.5. Statistical Analysis
The inferential statistics were used to predict the distribution of the experimental data by applying the Kolmogorov-Smirnov test (reference), which it defines whether the samples are parametric or nonparametric. This test will evaluate, at a significance level of 5%, if there are significant differences between CA-1 and CA-2 adsorbents using the results from the studies of adsorption kinetics and equilibrium of benzene and toluene.
Student t test (parametric samples) and Wilcoxon paired test (non-parametric samples) were applied.
3. Results and Discussion
3.1. Characterization of the Adsorbent
3.1.1. Specific Surface Area (SBET) and pH
Table 1 shows the results obtained for some of the physical properties of the adsorbents.
Adsorbent CA-1 showed basic pH of 8.9 whileacid treated CA-2 showed a pH value of 3.8. According to Villacanas et al [24] , the basic character of activated carbon results from the contribution of two factors. One is related to surface groups, such as pyrone and chromene and the other is associated with the existence of regions rich in π electrons of grafênicas layers, which act as Lewis base [25] . The activated carbons that have acidic surfaces are characterized by high oxygen contentcontaining functional groups such as carboxylic, phenolic and lactones.
The value of specific surface area is comparable to the values reported in the literature [26] (823.1 - 982.9 m2/g). Generally, the adsorption capacity increases with increasing specific surface area due to the availability of sites for adsorption [27] . According to Mangun et al [28] , the percentage of removal of BTEX in aqueous solution generally increases with increasing surface area.
It can be seen that the VP and SBET values are approximately the same for both adsorbents. In CA-2, the lowerin specific surface area is due to partial or complete blockage of oxygen in the complex pore wall [29] [30] . Similar results were reported [31] [32] .
3.1.2. Functional Groups on the Adsorbents Surface
Table 2 shows the results obtained for the determination of surface groups using the Boehm method.
There is a higher proportion of acidic groups in adsorbent CA-2 compared to CA-1 sample. This can be attributed to the preferential reaction of nitric acid with the basic groups (pyrones and chromenes) forming acidic groups by opening the heterocycles [33] . Acidification of the surface gives the activated carbon a higher ion exchange capacity and amphoteric character due to introduction of various functional groups on the surface [34] . According to Daifullah and Girgis [35] , the presence of carboxyl groups promotes increased adsorption capacity of activated carbon for BTEX.
3.1.3. Characterization of the Adsorbent Surface by Infrared Fourier Transform
Figure 1 shows the FTIR spectra of CA-1 and CA-2. The FTIR spectrum of the adsorbent CA-1showed bands
![]()
Table 1. Physical properties of adsorbents.
![]()
Table 2. Acidic and basic functional groups on the surface of activated carbon.
![]()
Figure 1. FTIR spectra of CA-1 and CA-2.
around 1100 - 1180 cm?1, 1550 - 1600 cm?1 and 2300 cm?1, which are attributed to stretching and vibration of CO group, stretch of C=O and presence of carboxylic groups and stretch of CH2 and CH3, respectively.
The peaks from 500 to 2000 cm?1 are assigned to carboxyl groups. The intense band at 1750 cm?1 is characteristic of the C=O stretch of carboxylic groups [36] -[38] . The intensity of the bands of CA-2 in the region that characterizes the carboxylic groups is greater than of CA-1 sample showing a largest concentration of these compounds after acid treatment. Similar results were found in other studies of adsorption of aromatic compounds by activated carbon [39] .
3.2. Results of Adsorption Experiments
3.2.1. Kinetics of Adsorption
Figure 2 show the percentage removal efficiency (ER,%) of benzene and toluene obtained from adsorption kinetics tests. Table 3 shows the parameters of the kinetic models used to correlate the experimental data.
The removal efficiency of benzene or toluene as a function of time, after 25 minutes of contact was greater than 90.0% in all tests.
The Kolgomorov-Smirnov test, we verified the normality of the data removal efficiency (ER,%) for the adsorption of benzene by both adsorbents. The parametric samples t test showed that both adsorbents differ statistically at 5% significance level.
For the adsorption kinetics of toluene, both adsorbents showed non-normality of the data removal efficiency. The adsorbents were statistically different at the 5% significant level.
![]()
Figure 2. Removal efficiency of benzene (a) and toluene (b) by CA-1 and CA-2.
![]()
Table 3. Kinetic parameters for the removal of benzene and toluene for the CA-1 and CA-2 adsorbents.
3.2.2. Adsorption Balance
Figure 3 shows the experimental data having applied Langmuir mathematical model for the CA-1 and CA-2. Table 4 shows the parameters calculated using the model. From the results showed that the data perfectly fits the Langmuir mathematical model.
The maximum adsorbed benzene and toluene amount, according to the Langmuir model (Qmax) was around 6.0 mg/g for the two adsorbents. RL values were within the range (0 < RL < 1), which according to Ho et al. [40] and Dizge et al. [41] indicate favorable adsorption type of benzene and toluene by both adsorbents. Similar results were found by Daifullah and Girgs [35] in their work on BTEX adsorption using activated carbon.
This experimental data one show that there is no significant difference in the adsorption of benzene and toluene using these adsorbents.
3.2.3. Adsorption in Fixed Bed
Figure 4 and Figure 5 show the rupture curves for the adsorption of benzene and toluene (Figure 5) at the optimum volumetric flow rate (70 ml/min) using CA-2.
![]()
Figure 3. Data adsorption of benzene (a) and toluene (b) adjusted by Langmuir model.
![]()
Table 4. Linearised langmuir parameters for the adsorption of benzene (a) and toluene (b) CA-1 and CA-2.
The breakpoints were around ce/c0 values of 0.076 and 0.079 for benzene and toluene respectively, in 10 minutes. The organic percentage were around 92% for both adsorbents. The residual concentration of benzene and toluene were 4.7 and 4.5 mg/L respectively, higher than the maximum values allowed by Brazilian legislation for these organic compounds (0.05 and 0.17 mg/L) [42] .
Considering the experimental conditions used in this work (bed height, particle size of the adsorbent, the column internal diameter and volumetric flow rates of feeding) were not sufficiently adequate for the complete removal of benzene and toluene from aqueous effluents. The process become more efficient, if the inside diameter and the height of the bed is lower [34] . Under such conditions the losses by volatilization of organic compounds are lower.
![]()
Figure 4. Rupture curve for the adsorption of benzene.
![]()
Figure 5. Rupture curve for the adsorption of toluene.
4. Conclusions
The treatment of commercial charcoal by HNO3 was effective because there was an increase in the concentration of surface acid groups, particularly carboxyl groups.
However, based on the experimental data one can say that there was no significant quantitative difference in the adsorption of benzene and toluene from the activated carbon treated (ca-2) in relation to in nature (ca-1). Having necessity for further studies to optimize experimental conditions of adsorption experiments, as well as adsorbents which can remove more efficiently organic compounds (BTEX) from industrial wastewater, surface water and groundwater in order to minimize the toxic effects of these pollutants to the environment and communities affected by them.
Acknowledgements
Graduate Program in Chemical Engineering from the Federal University of Pará (UFPA-PPEQ); Capes-CNPq; Laboratory of Toxicology―Environment Section―InstitutoEvandroChagas/Para/Brazil.