Hydrolysis of Trivalent Holmium in Aqueous Solutions of 2 M Ionic Strength by Spectrophotometric and Potentiometric Methods ()
1. Introduction
The behavior of Ho3+ ions in aqueous solution is of increasing interest because the holmium is an excellent radiolanthanide have a range of half-lives and beta energies suitable for radiotherapy [1] and is analogue with Einsteinium, because the lanthanides are used as analogues for radioactive trivalent actinides [2] . Producing and accumulating of significant quantities of lanthanides during nuclear fission in uranium and Plutonium reactors are a potential source of their formation [3] . The knowledge of the hydrolysis constants of these elements allows the comprehensive modeling of radionuclide transport around proposed radioactive waste storage [4] . The deposition of non-or radioactive wastes in subsurface repositories of salt beds or ocean can be produce complexation with hydrofilic ligand, because complexation strongly affects their mobility [2] . This is the reason for employing the ionic strength of NaCl and compare with the use of NaClO4.
The hydrolysis constants of holmium III are scarce; published data have been by determined by potentiometric titration, solvent extraction and spectrophotometric titration [5] - [7] .
The scatter of data of the stability constants of the hydroxo forms of trivalent holmium element in aqueous solutions. This circumstance is reasonable for the wide scatter of experimental stability constants, which was ob- served even for a single element at the same ionic strength [7] .
The pM(aq)-pCH diagram allows for the determination saturated and unsaturated zones in a solution, also allows for the calculation of the pCH of precipitation (pCHpp) and hydrolysis constants [8] - [11] .
The fundamentals of hydrolysis of rare earth elements and the pM(aq)-pCH diagram are found elsewhere [8] - [10] and only the main definitions being included here. At the initial, the first hydrolysis constant is calculated using:
 (1)
(1)
Considering the influence of a 2 M NaCl medium on hydrolysis equilibrium, the log10β1,Cl values might be calculated from the hydrolysis constant values obtained in both the absence and the presence of chloride ions at the same ionic strength and temperature. In a solution of chloride ions as high as 2 M [11] :
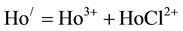
Then
 (2)
(2)
and
 (3)
(3)
Finally
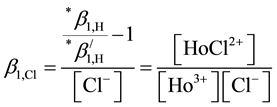 (4)
(4)
The determination of metal concentration in aqueous solutions by spectrophotometric and potentiometric me- thods can be used to calculate the equilibrium constants of dissolved elements in aqueous solutions, because the IUPAC recommends using two methods or more [2] . The spectrophotometric method allows for the determination the pM(aq)-pCH diagram, calculation of the pCH of precipitation (pCHpp), and hydrolysis constants and potentiometric method using saturated and unsaturated zones determinated with the pM(aq)-pCH diagram. In addition, computer programs such as SQUAD (stability quotients from absorbance data) [12] and SUPERQUAD [13] have been developed to elucidate solution equilibria. All non linear least-squares methods (SQUAD and SUPERQUAD) are based on the minimization of function U, given by Equation (5):
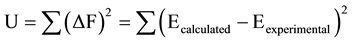 (5)
(5)
The input file data for SQUAD and SUPERQUAD includes the pH measured, the metal concentration, and a chemical model that includes stoichiometry and estimated values of formation constants of the species present during titrations. The difference between these programs is that SQUAD utilizes the UV/Vis absorbance values, while SUPERQUAD utilizes the cell potential or pH. Both programs provide the refined values of formation constants, as well as a very complete statistical analysis of the data.
In a previous study it has reported results of investigation of the hydrolysis of La, Pr, and Lu in aqueous solutions using potentiometric method and different treatment of the data [11] . Hydrolysis and the stability constant of the chloride species of lanthanum, praseodymium and lutetium were determined for each element; all these studies were carried out in 2 M sodium perchlorate and sodium chloride in a nitrogen atmosphere and at temperature of 303 K.
This paper extends the study of the rare earth elements (REE) to holmium and, by using UV-Visible spectroscopy and potentiometric methods, permits reliable comparison of the hydrolysis of Ho with that of La, Pr, and Lu. Here we report the results of an experimental investigation of the speciation of Ho in solution at temperatures of 303 K.
The aim of the present research was to explore the influence of chloride ions on the solubility product of solid hydroxides and the first hydrolysis constants of trivalent holmium. The experimental conditions were 2 M ionic strength, imposed with sodium perchlorate or chloride. The temperature was controlled at 303 K and the methods used were spectrophotometric and potentiometric. Data obtained were treated with both the program SQUAD and SUPERQUAD.
2. Experimental
All reagents were of analytical grade, and boiled deionized water being used to prepare the solutions. All prepared aqueous solutions contained either 2 M NaClO4 or NaCl. Holmium (III) nitrate pentahydrate [Ho(NO3)3-5H2O], 99.9% Sigma-Aldrich Co. M.W. = 382.56 g/mol was dissolved in 10−3 M hydrochloric acid. The concentration of holmium in the standard solution was determined by titration with 0.025 M EDTA solution. Finally, three drops of pyridine and three drops of xylenol orange were added as an indicator [10] . The holmium concentration in the standard solution was 0.25 M. Starting from this solution, other solutions were made by dilution to obtain the calibration curves of holmium solution by UV-Vis spectrophotometry and for spectrophotometric titration.
For the preparation of the hydrochloric acid solutions, we started from HCl 1 M (Merck, titrisol). Starting from this solution, all the others were prepared by dilution.
The standard solutions of sodium hydroxide were prepared in a glove box under a nitrogen atmosphere, by diluting the supernatant from a 50% w/v sodium hydroxide aqueous solution which had been centrifuged to separate the insoluble sodium carbonate [14] . The titration of the standard solutions with hydrochloric acid proved that they were free of carbonate ions. These solutions were used immediately, or were stored in the same glove box under a nitrogen atmosphere. The lids of the solution bottles had a trap containing ascarite as a CO2 absorbent. The concentration of sodium hydroxide of each stock solution was determined by titration with potassium hydrogen phthalate, the ionic strength being adjusted with NaCl or NaClO4.
2.1. Determination of the pCH-pH Relationship at NaCl 2 M or NaClO4 2 M
Spectrophotometer and potentiometric calibration lines were obtained of solutions of 0.1, 0.01, and 0.001 M sodium hydroxide and hydrochloride acid was prepared in 2 M sodium perchlorate or sodium chloride ionic strength, as described elsewhere [10] , and all pH measurements were corrected by means of the equations ob- tained:
 (6)
(6)
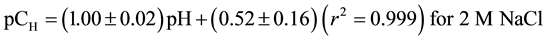 (7)
(7)
where r is the correlation coefficient.
The pH measurements were carried out with a combined electrode system (glass-AgCl/Ag), which has low interference coefficients for alkali ions. A pH-meter (Radiometer TIM900 Titrilab), together with an automatic buret (Radiometer ABU901), a double-walled cell, and a constant-temperature circulator (Polyscience Circulator 12101-10), were used to measure the pH with a precision of 0.001 pH units. All the experiments were carried out at (303.0 ± 0.1) K, and a nitrogen flux was maintained in the reaction cell. The pCH of sodium perchlorate and sodium chloride stock solutions were adjusted to 3 with hydrochloric acid in order to prevent the hydrolysis of holmium element before the experiments began.
2.2. Calibration Curves of Holmium Solution by UV-Vis Spectrophotometry
To obtain the absorption calibration curves, 10 cm3 solutions of 0.125, 0.0625, 0.025, 0.01, 0.005, 0.0025, 0.001, 0.0005, 0.00025, 0.0002, and 0.0001 M (pH = 3) were prepared using the 0.25 M holmium standard solution. The absorbance spectra of the resulting solution were obtained in the range of 200 - 700 nm using a Perkin-El- mer UV/Vis Lambda 10 spectrophotometer (Fremont, CA).
The data treatment was processed with an Excel worksheet (Microsoft), to determine the relation absorbance in function to the wavelength.
2.3. First Hydrolysis Constant Determination of Ho (III) by Spectrophotometric Method
The photometric titrations were obtained by following a previously described methodology [2] . Briefly, a known volume of the 6.25 × 10−2 M Ho solution (initial pH = 3) was transferred into a titration double wall cell containing 20 cm3 of the 2 M NaClO4 or NaCl solution both at initial pH = 3 to prevent holmium hydrolysis. The transferred volume was enough to obtain a final Ho concentration of 2.5 × 10−4 M. The initial solution was stir- ed for thirty minutes before the spectrophotometric titrations started and nitrogen flux was kept on the solution during the experiment, after which the solution’s pH was recorded. Subsequently, an aliquot of 3 cm3 was taken from the reaction double wall cell to determine the UV-Vis absorption, and the volume obtained was returned to the titration double wall cell. After that, a known volume of the 2.5 × 10−3 M NaOH in 2 M NaClO4 or NaCl was added to the titration cell. After equilibration for 7 minutes, another aliquot was taken to determine the new pH and absorbance. The absorbances versus wavelength profiles of the resulting solutions were obtained in the range of 200 to 700 nm. Addition of the NaOH solution, pH, and absorbance measurement was repeated until the pH was about 10. A nitrogen flux was maintained on the solution during the experiments. At least two experiments were performed under the same conditions to obtain reproducibility. The experimental values of the pCH, absorbance, and volumes of NaOH added in each titration point, were analyzed using the SQUAD [12] computer program, as well as the graphic method [2] . The absorbance versus pCH data were plotted using the Excel®.
2.4. First Hydrolysis Constant Determination of Ho (III) by Potentiometric Method
The experiments for the potentiometric titrations were carried out as follows: The concentrations of the solutions of holmium were 2.5 × 10−4 M and 2.5 × 10−3 M for sodium hydroxide in 2 M sodium perchlorate or sodium chloride. The initial volume in the titration double wall cell was 20 cm3 of 2 M NaClO4 or NaCl both at pH = 3. The initial solution was stirred for thirty minutes before the potentiometric titrations started and nitrogen flux was kept on the solution during the experiment. The added volume of the sodium hydroxide solution (the aliquot volume was 20 mm3) and the pH measurements were recorded. The pCH values were calculated by the equations given above. The experiments were carried out at 303 K and at least three experiments being performed under the same conditions to analyze reproducibility. The Data were mathematically treated with the program SUPERQUAD in order to calculate the equilibrium constants.
3. Results and Discussion
3.1. Absorbance versus Concentration Curves
The UV-Vis absorbance vs. concentration curves of holmium standard solutions were obtained in the range 255 to 660 nm at pH 3 (Figure 1). Spectra for concentration ranging from 0.0001 M and 0.0002 M were not well defined and therefore discarded. In the present work, the lower detection limit was 0.00025 M. The concentrations ranging of the spectra were from 0.00025 to 0.25 M. Spectra have 11 wavelengths (301, 345, 361, 386, 417, 451, 468, 473, 485, 537, and 641 nm). It can be noted that in the UV region (255 to 330 nm) there is a wide band between 0.00025 M £ [Ho(III)] £ 0.125M. For the absorption band of 301 nm (Figure 1), is to determine low
![]()
Figure 1. UV-Vis absorption spectra for each one of the solution of holmium (III) at pH 3, in the range 255 - 660 nm.
concentration of holmium. On the other hand, we can determine high concentration of holmium in narrow absorption bands (346, 386 principally, 468, and 473 nm) are observed between 0.0625 M £ [Ho(III)] £ 0.25 M (Figure 1).
These results extend the range of holmium concentration that can be determined and are shown in Table 1.
Theses equations will permit to extend the range to calculate concentration of holmium, but have a limitation as it should be noted that the optimal range for measuring the absorbance for the purpose of quantitative analysis, is between 0.02 - 1.5.
3.2. Spectrophotometric Method
The values of the hydrolysis constants of Ho(III) were determined using UV-Vis titration in a CO2 free atmosphere at 303 K. Figure 2 shows the UV-Vis absorption spectra of the titration of 0.00025 M Ho(III) with 0.0025 M NaOH carried out in 2 M NaClO4 or NaCl at different pCH values. Each curve in Figure 2 represents the UV- Vis absorption spectrum of the Holmium solution at each titration pCH. The UV-Vis absorption spectra overlapped in the regions of 301 nm.
The absorption values in the range 280 to 325 nm, the solution pCH (in the range 2.78 to 7.01 pCH, and the wavelength of 301 nm in 2 M NaClO4 are found and shown in Figure 2. These values in 2 M NaClO4, the range 4.36 to 6.80 pCH in 2 M NaCl, and the Ho(III) concentration were analyzed using the SQUAD computer program, as well as the graphic method.
3.2.1. pHo(aq)-pCH Diagrams
The pHo(aq)-pCH diagram (the absorbance-pCH curves) for holmium in 2 M NaCl or 2 M NaClO4 at 303 K are shown in Figure 3. The absorbance of holmium in 20 cm3 of 2.5 × 10−4 M holmium solution was determined using a perking-Elmer UV-Vis Lambda 10 spectrophotometer (Fremont, CA).
Moreover, changes of holmium concentration because of the addition of sodium hydroxide solution must also be considered. Figure 3 shows that the experimental data follow the behavior predicted by the mentioned equation up to a certain pCH value. The inflection indicates the borderline between unsaturated and saturated zones.
This figure shows that the pCH for the precipitation beginning are 7.17 ± 0.14 in 2 M NaCl and 6.80 ± 0.05 2 M NaClO4 respectively (Table 2). Although the diagrams obtained in both ionic strength were similar, a sudden increase of pHo(aq) was observed at lower pCH values.
At this wavelength was calculated the first hydrolysis constant of holmium using the derivate graph, of its first derivative from absorbance-pCH curve. The maximum of the first derivative indicates an estimator of log*β1 value [15] , because it signals the inflection point of the sigmoid absorbance-pCH curve. The estimator obtained by this graphic method is log*β1 = −8.16 ± 0.02 in 2 M NaCl and log*β1 = −8.02 ± 0.01 in 2 M NaClO4, respectively.
In this work, pHo(aq)-pCH diagrams were obtained from data corresponding to freshly precipitated holmium hydroxides.
![]()
Figure 2. UV-Vis absorption spectra for the titration of holmium (III) 0.00025 M with NaOH 0.0025 M in 2 M NaClO4 at 303 K.
![]()
Figure 3. Curve of absorbance as a function of pCH, in 2 M NaClO4 and 2 M NaCl at 303 K, for wavelength of 301 nm. The first derivative of this curve is represented with the solid line.
3.2.2. Determination of the Hydrolysis Constants of Holmium(III) by SQUAD
The computer program SQUAD [12] was used to confirm the first hydrolysis constant of Ho(III), as previously calculated with the pHo-pCH diagram. SQUAD calculates overall stability constants values by mean of a nonlinear least-squares approach. The data for SQUAD calculations are: UV-Vis absorption data, Ho(III) concentration, and a chemical model to describe the system (i.e. ). The hydrolysis constants of
). The hydrolysis constants of
Ho(III) obtained using SQUAD were 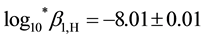 in 2 M NaClO4 and
in 2 M NaClO4 and  in 2 M NaCl (Table 2). The hydrolysis constants of Ho(III) calculated using SQUAD were very similar to the hydrolysis constants calculated using the pHo-pCH diagram in the same ionic strength [2] [10] [11] , and different when ionic strength is different [11] .
in 2 M NaCl (Table 2). The hydrolysis constants of Ho(III) calculated using SQUAD were very similar to the hydrolysis constants calculated using the pHo-pCH diagram in the same ionic strength [2] [10] [11] , and different when ionic strength is different [11] .
3.3. pH Titration
Figure 4 shows typical curves of pH titrations for holmium carried out in 2 M sodium perchlorate and 2 M sodium chloride. The data range used to feed the program SUPERQUAD was selected as follows: the initial point was taken immediately after the excess of acid was neutralized, i.e. where the first inflection of the titration curves was the highest. On the other hand, the end point was selected just before Ho(OH)3 precipitation started, according to the pHo(aq)-pCH diagrams. As mentioned above, all these determinations were carried out using a set of equipment.
![]()
Figure 4. Typical titration curves of homium in 2 M NaClO4 and 2 M NaCl ([Ho3+]initial = 2.5 ´ 10−4 M; [NaOH] = 2.5 ´ 10−3 M). First derivative lines are included.
![]()
Table 1. Beer-Lambert law ranges of validity for the UV-Vis absorption bands of holmium(III) at pH 3.
Where: nm = nanometer, A = Absorbance, [Ho(III)] = holmium concentration, R2 = correlation coefficient, m = slope, Sy = Linear regression standard error.
![]()
Table 2. pCH borderlines of precipitation, first hydrolysis constants in molarity units for holmium (III), in 2 M aqueous NaClO4 (log10*β1,H) and 2 M aqueous NaCl ![]() at 303 K, from (A) pHo(aq)-pCH diagrams, (B) spectrophotometric titration and SQUAD program,(C) pH titration and SUPERQUAD program. Mean values of these calculations are also included (D).
at 303 K, from (A) pHo(aq)-pCH diagrams, (B) spectrophotometric titration and SQUAD program,(C) pH titration and SUPERQUAD program. Mean values of these calculations are also included (D).
Determination of the Hydrolysis Constants of Holmium(III) Using SUPERQUAD
The computer program SUPERQUAD [13] was used to confirm the hydrolysis constants of Ho(III). The data for the SUPERQUAD calculations are Ho(III) concentration, volume of NaOH added at each point in the system, the pCH measured, and the chemical model to describe the system given above.
The hydrolysis constants of Ho(III) obtained using SUPERQUAD were ![]() in 2 M NaCl, and log10*β1,H = 8.02 ± 0.07 in 2 M NaClO4 (Table 2).
in 2 M NaCl, and log10*β1,H = 8.02 ± 0.07 in 2 M NaClO4 (Table 2).
Table 2 shows the Brönsted first hydrolysis constants for holmium in 2 M sodium perchlorate and 2 M sodium chloride, which were calculated by taking Kw as a constant (log10Kw = −13.72 for 2 M NaClO4 and log10Kw = −13.68 for 2 M NaCl). Statistical parameters of the SUPERQUAD program were stotal between 1.99 and 16.01 and c2 4.0 and 10.0, in both case.
As shown in Table 2, the hydrolysis constants of Ho(III) calculated with SUPERQUAD was very similar to the hydrolysis constants calculated using the computer program SQUAD [12] and the pHo(aq)-pCH diagram [8] - [12] in both ionic strength.
As describe elsewhere [11] , data of Table 2 show that for a given element![]() , this behavior can be explained by the high concentration of chloride ions present in these solutions.
, this behavior can be explained by the high concentration of chloride ions present in these solutions.
The same table shows that [11] , the pCH borderline of precipitation is lower in NaClO4 than in NaCl solution. This behavior can be explained by the formation of the soluble HoCl2+ specie in the system, due to the high concentration of chloride ions when working in 2 M NaCl. Chloride ions compete with hydroxyl ions for the com- plex formation with rare earth elements and the pCH borderlines of precipitation are thus higher in the presence of chloride ions than in their absence [11] .
The first hydrolysis constant for Ho(III) (log10*β1,H = −8.01 ± 0.05 and![]() ) calculated in 2 M NaClO4 and 2 M NaCl ionic strength and with the data reported elsewhere [11] (log10*β1,H = −8.63 ± 0.05 and
) calculated in 2 M NaClO4 and 2 M NaCl ionic strength and with the data reported elsewhere [11] (log10*β1,H = −8.63 ± 0.05 and ![]() (La), log10*β1,H = −8.37 ± 0.02 and
(La), log10*β1,H = −8.37 ± 0.02 and ![]() (Pr), log10*β1,H = −8.00 ± 0.5 and
(Pr), log10*β1,H = −8.00 ± 0.5 and ![]() (Ho), and log10*β1,H = −7.95 ± 0.07 and
(Ho), and log10*β1,H = −7.95 ± 0.07 and ![]() (Lu)) follows the same behavior along the lanthanide series, that is, the log10*β1,H and
(Lu)) follows the same behavior along the lanthanide series, that is, the log10*β1,H and ![]() increase with atomic number [11] . As observed, the presence of chloride ions and their concentration are important parameters in the determination of the hydrolysis constants.
increase with atomic number [11] . As observed, the presence of chloride ions and their concentration are important parameters in the determination of the hydrolysis constants.
For a comparison of the values obtained in the present research with others reported previously, the constants should be referred to the same conditions. For example (Table 3), Frolova et al., obtained a value of the logβ1,H of −9.96 [5] , in 0.3 M NaClO4 y 0.02 M Ba(OH)2 by potentiometric titration, Guillamount et al. [6] , a value of the logβ1,H of −5.7, in 0.1 M LiClO4 by solvent extraction, and Stepanchikova and Biteikina [7] a value of the logβ1,H of −8.15 in zero ionic strength by potentiometric titration, they all at 298 K. These values are of different magnitude as that reported in this paper, but is of the same order of magnitude as that the Stepanchikova and Biteikina, although ionic strengths and temperatures are different.
3.4. The Stability Constants of the First Chloride for Holmium Specie
Chlorides ions compete hydroxyl ions more strongly for formations with Ho(III) because the influence of a 2 M NaCl than in 2 M NaClO4. The log10β1,Cl value was calculated by using the first hydrolysis constants obtained in 2 M NaCl and 2 M NaClO4 and Equation (4). Table 4 shows the values obtained in this research and those
![]()
Table 3. Literature data of the first hydrolysis constant of holmium.
P: potenciometric; SE: solvent extraction; SP: spectrophotometric.
![]()
Table 4. Comparison of stability constants of HoCl2+ (M−1), taken from the literature and obtained in the present research.
E: estimated; P: pH titration; SP, spectrophotometric titration.
found in the literature [16] - [18] . Experimental conditions and the value obtained here are different as those reported by Wood [16] , Millero [17] and Hass et al. [18] .
The stability constant for the species HoCl2+ (log10β1,Cl = −0.56) calculated in 2 M NaCl ionic strength and with the data reported elsewhere [11] (log10β1,Cl = −0.0255 (La), −0.155 (Pr), −0.56 (Ho), −0.758 (Lu)) follows the same behavior along the lanthanide series, that is, the log10β1,Cl value decrease with atomic number [11] .
4. Conclusion
The hydrolysis constants (log*β1,H and![]() ) of holmium determined by the two methods were similar. The difference between the values of log*β1,H and
) of holmium determined by the two methods were similar. The difference between the values of log*β1,H and![]() , allowed to determine the value of logβ1,Cl of HoCl2+ in
, allowed to determine the value of logβ1,Cl of HoCl2+ in
2 M NaCl, showing thus the influence of chloride ions in the ionic strength of this research. Finally, the values of
the hydrolysis constant (log*β1,H and![]() ) and stability constant for the species with chlorides (logβ1,Cl) of
) and stability constant for the species with chlorides (logβ1,Cl) of
the Holmium, follow the same trend to those of La, Pr, and Lu reported previously.
NOTES
*Corresponding author.