1. Introduction
Consolidation and conservation of calcareous materials required products that were compatible with components originally used. Lime represents one of the best compatible consolidating and protective material given that, reacting with CO2 present in ambient air; it was converted to calcium carbonate [1] -[3] . However, commercial lime products suffered of some drawbacks: incompleteness of carbonatation process, reduced penetration depth and formation of a white film on treated surfaces [4] -[7] . These drawbacks were overcome by nanolime, i.e. Ca(OH)2 nanoparticles hydro-alcoholic suspensions, successfully employed in Cultural Heritage conservation and offering advantages in stone, mortar and plaster consolidation [8] -[14] . Nanolime, thanks to the reduced size, can penetrate into damaged zones and guarantee fast and complete reaction with CO2, mainly when exposed at relative humidity conditions higher than 75% [12] [14] -[17] .
As reported in literature [16] [18] , nanolime was obtained by a chemical precipitation process, in supersaturated conditions, by mixing drop by drop equal volumes of sodium hydroxide (NaOH) and calcium chloride (CaCl2) aqueous solutions, both maintained at high temperature (90˚C). Supersaturation degree, high temperature and slow mixing tended to favor Ca(OH)2 nucleation rate respect to particle growth, promoting the precipitation of nanosized calcium hydroxide particles, according to the following reaction:
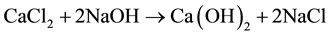 (1)
(1)
Several washings with deionized water were then necessary to remove the sodium chloride formed during the synthesis process. During each washing, some Ca(OH)2 particles were dissolved, particularly those with size < 100 nm, as they were more readily soluble, leading also to a very low yield in the production of nanolime particle. In order to reduce time of synthesis, an alternative method was proposed by simply adding a surfactant agent in the initial aqueous solutions [19] . Nevertheless, this method presented same drawbacks in relation to washings procedures to remove NaCl and surfactant too. Nano-sized Ca(OH)2 particles were also synthesized by hydrolyzing CaCl2 (or Ca(NO3)2) solution in diols and by adding the aqueous Na(OH) solution at more elevated temperature (up to 175˚C). Nevertheless, diols remained adsorbed onto the nanoparticles, so causing their aggregation and forming micron-sized agglomerates; this problem was overcome by peptizations with 2-propanol in an ultrasonic bath [20] [21] .
In order to have better reactivity [10] and to reduce the tendency for a white film on surfaces to be consolidated [4] , nanolime suspensions, whatever produced, were finally dispersed into an alcoholic medium, (i.e., 2- propanol), to limit particles agglomeration, thus lowering sedimentation rates and allowing a higher suspension stability. The synthesis procedures described above had main drawbacks: 1) slow mixing rates; 2) the necessity to separate and to remove secondary products or undesired phases (by water washings or peptizations), that can cause the loss of calcium hydroxide dissolved in water [22] ; 3) low specific yield of Ca(OH)2 nanoparticles production.
In this paper an innovative and original patented method [23] , based on the use of an anionic exchange resin (in OH− form) was proposed, that allowed producing, in few minutes and without critical separation steps, pure and crystalline Ca(OH)2 nanoparticles. Kinetic of the exchange process was analysed, in order to find the optimal R/C ratio, (where R was the total resin capacity and C was the total equivalent mass of the chlorides in solution), able to guarantee the best results in terms of Ca(OH)2 production. Regeneration of the exhausted resin was also investigated, in order to determine the NaOH consumption.
Preliminary characterization analyses were performed, by means of transmission electron microscopy (TEM) and X-ray diffraction measurements (XRD). Carbonatation process in ambient air of the Ca(OH)2 nanoparticles suspensions, at different water/alcohol ratios, was estimated too. The results were compared with those obtained on nanolime particles produced by a typical drop by drop method, performed in aqueous solutions.
2. Experimental Section
2.1. Materials
Materials used to produce nanolime were calcium chloride dihydrate ³ 99% (CaCl2⋅2H2O), supplied by Merck, anion exchange resin Dowex Monosphere 550A (OH), with a total volume capacity of 1.1 eq/l, 2-propanol > 99.8% (Merck), deionised water (purified by a Millipore Organ0ex system (R ≥ 18 MW∙cm).
2.2. Synthesis of the Ca(OH)2 Nanoparticles
Ca(OH)2 nanoparticles were synthesized, at room temperature, by mixing under moderate stirring the anion exchange resin with the aqueous calcium chloride solution. When stirring was stopped, resin was separated from the precipitate by using a sieve (“one step process”), and put in a closed bottle. The residual concentration of chlorides in the nanolime suspension can be further lowered down, to a desired value, by putting in contact the produced suspension of nanolime with a fresh ion exchange resin, also producing additional nanolime particles (“two steps” process). The experimental details of the “One step” and “Two steps” process were shown below.
“One step” process. An aqueous solution containing 0.1 M of CaCl2⋅2H2O was put in contact with 75 ml of resin, at room temperature and under moderate stirring for 60 minutes. Then, we have prepared other two suspensions, starting with the same CaCl2⋅2H2O solution but considering different resin amounts (115 ml and 140 ml of resin volume, respectively). In summary, three suspensions were obtained by different R/C ratios (that is, 1, 1.5 and 1.8), and from here named WR1, WR2 and WR3, respectively.
“Two steps” process. An aqueous solution containing 0.1 M of CaCl2∙2H2O was put in contact with 115 ml of resin (that is, the resin volume giving the best R/C ratio, as it will be shown later on), at room temperature and under moderate stirring for 5’. The suspension was separated from resin by a sieve (180 mm), put in contact with 115 ml of a fresh resin. The preparation was then maintained under stirring for 60’ and finally separated. A suspension named WR2b was obtained.
We have compared the nanolime suspensions obtained by the new synthetic route with one produced “drop by drop”, as reported in literature [16] [24] . In particular, it was obtained by adding dropwise 0.6 M Na(OH) solution, at a rate of 4 ml/min, to 0.3 M CaCl2⋅2H2O one, both maintained at 90˚C. Five washings with deionised water were necessary to remove NaCl, causing the loss of about 30% of the produced Ca(OH)2. The final suspension was named W0.
All the synthesized suspensions were characterized by a nanolime concentration of about 8 mg/ml.
As reported in literature [8] [10] , in order to obtain more stable suspensions, we partially substituted water with an alcoholic solvent (2-propanol). The hydro-alcoholic suspensions so obtained were characterized by a water/2-propanol ratio (W/A) ratios of 100%, 75%, 50% and 10% respectively.
2.3. Analysis of Chlorides Concentration
In order to evaluate the resin exchange kinetics, that is the speed with which OH− and Cl− exchange takes place, some samples were taken from the preparation under stirring, at different times, from the beginning of the synthesis up to 60 minutes of stirring. By an ion selective electrode (Metrohm) we have measured chlorides content in the initial CaCl2⋅2H2O aqueous solution (C0) and chloride concentration measured at time t (Ct).
2.4. Resin Regeneration
Exhausted anion exchange resin was regenerated in a column by using a 1.5 M NaOH aqueous solution, at a flow rate of 20 ml/min. The resin regeneration was regulated by following reaction:
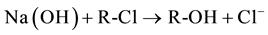
At the end of the regeneration, the chlorides concentration of spent Na(OH) regenerated solution was analyzed by ion selective electrode (Metrohm) in order to evaluate the regeneration level. After the regeneration process, the regenerated resin can be reused for production of Ca(OH)2 nanoparticles, by considering the same procedure used for WR2 sample. The suspension produced with the regenerated resin was so called WR2r, evaluating, as before, resin exchange kinetic.
2.5. Ca(OH)2 Nanoparticles Characterization
In order to analyse morphology and particles dimension, the synthesised suspensions were characterized by TEM technique, dispersing 0.2 ml of each nanolime suspension in 2-propanol and, after some minutes in US, some drops were deposited on a carbon-coated copper grid.
Crystal phases that formed during the production process were analyzed by X-ray diffraction technique (PANalytical X’Pert diffractometer, CuKa radiation). Besides, this technique allowed us to evaluate the particles reactivity by following the carbonatation process of Ca(OH)2 particles in air, according following steps:
- carbon dioxide was dissolved in water 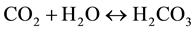
- Ca(OH)2 reacted with carbonic acid 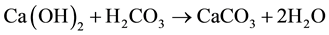
To investigate carbonatation process, we have maintained each nanolime suspension for 20’ in ultrasonic bath (US); then, 0.2 ml of the suspension was deposited on a XRD zero background sample holder, and left in air, in ambient conditions, (T = 20˚C, R.H. 50%, pCO2 ~10−3.5 atm, i.e. the standard concentration of CO2 in air) until solvent was evaporated. Measures were performed on dry sample.
XRD patterns were recorded in the angular range from 5˚ to 60˚2θ, in steps size of 0.026˚2q. Each experimental diffraction pattern was elaborated by a Profile Fit Software (HighScorePlus, PANalytical), and each crystalline phase was attributed by ICSD and ICDD reference databases. The ratio between the CaCO3 peaks area and the pattern total area was assumed as the carbonatation process efficiency (χ).
Moreover, XRD measures of alcoholic suspension carbonatation process (W/A = 10%) and exposed to a high humid condition (RH = 90%), were performed too. XRD spectra were acquired on dry sample, after different air exposure times (1, 5, and 7 days, respectively).
3. Results and Discussion
Following the proposed synthetic route, pure Ca(OH)2 nanoparticles should be obtained according to the reaction:
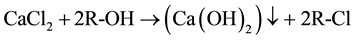 (2)
(2)
The equilibrium conditions can lead to a residual concentration of chlorides in the nanolime suspension. Moreover, since a reduction in chlorides concentration should be related to the Ca(OH)2 production, the chlorides percentage decrease (% ΔC), defined as:
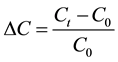
was useful to estimate, by stoichiometric calculation, the Ca(OH)2 produced at different times. Besides, C0 can be used to estimate the maximum theoretical value of Ca(OH)2 (CHmax) that should be obtained from the synthesis process.
In particular, in Table 1, Ct values and Ca(OH)2 production from kinetics measurements (“One step” and “Two steps” process) were reported, after t = 5’, 30’ and 60’ (minutes) respectively, from the beginning of synthesis process. Measures denoted a very fast exchange process rate; in fact,, after only 5’, it can be observed that WR2 and WR3 suspensions had a Ca(OH)2 production higher than 95% of the maximum theoretical value (CHmax) and a residual chlorides concentration extremely reduced (Ct ranging from 280 to 335 mg/l). Besides, suspension synthesized by the “Two steps” process (WR2b suspension) allowed obtaining, after 5’ of stirring with the second resin, a nanolime suspension containing a reduction of chlorides of more than 99.8%, with a limit chlorides concentration of 12 mg/l.
From Table 1 we have also established that the best conditions for the production were obtained in WR2 sample, unless very low values of chlorides are required. In example, if we consider typical lime applications, as those in architectural fields, acceptable residual chloride concentration ranges from 4500 mg/l for unreinforced concrete up to 500 mg/l for restoration mortars [25] , so WR2 suspension meets the standards for chloride concentration and does not need the additional treatment to reduce the chlorides concentration itself. As concerns WR2r suspension, that is the one prepared by the regenerated resin, we have verified that the regeneration process was very efficient for a new and comparable Ca(OH)2 nanoparticles production.
XRD analyses were performed to determine crystalline phases present in suspension. From XRD patterns we observed that Ca(OH)2 was the only phase existing in each suspension. As an example, WR2 pattern was reported in Figure 1, in comparison to W0 sample. Besides, as observed in previous works [10] [19] [20] , the dried Ca(OH)2 nanoparticles tend to align in a preferential direction along the basal plane (001).
The presence of Ca(OH)2 was also confirmed from TEM images, which showed the typical hexagonal habitus of calcium hydroxide, as observed in Figure 2. In particular, TEM images referred to WR2 suspension showed nanoparticles of side dimension generally less than 100 nm. In Figure 2(b)) a group of nanoparticles, with dimensions ranging from 80 nm to less than 40 nm, was shown; a nanoparticle of rhombohedra morphology can
![]()
![]() (a) (b)
(a) (b)
Figure 1. (a) XRD pattern of W0 suspension; (b) XRD pattern of WR2 suspension.
![]()
![]() (a) (b)
(a) (b)![]() (c)
(c)
Figure 2. (a) (b) TEM micrographs on WR2 sample. (a) single small particles, hexagonally plated, with side dimension of about 50 nm; (b) a group of Ca(OH)2 particles (side dimension ranging from 80 nm to less than 40 nm) overlapped in a ordered way and transparent to the electron beam. (c) TEM micrograph on W0 sample: Ca(OH)2 particles hexagonally plated and regularly shaped with side dimension up to 400 nm [28] .
![]()
Table 1. Chlorides concentration at different time intervals (Ct) measured from suspension by ion exchange process, after different times, t (5’, 30’ and 60’, respectively).
*Values obtained after the mixing with the second resin.
be attributed to the formation of calcium carbonate in form of calcite. These results were comparable, in terms of particles dimensions, with those typically reported in literature and obtained after several steps of purifications and at higher temperature (up to 175˚C) [20] [24] . On the contrary, TEM micrographs referred to nanolime particles synthesized according to “drop by drop” method (W0 suspension) were hexagonally plated, but with side dimension up to 200 nm (Figure 2(c)), in accordance with those reported in literature ([14] and ref in).
XRD measurements were performed to evaluate the carbonatation process of Ca(OH)2 suspensions too. We have observed that nanolime suspensions produced by exchange process, when exposed in ambient air, carried out to the formation of calcium carbonate in form of calcite. In Table 2 we have reported the yield values χ, referred to WR2 and W0 suspensions, by considering different W/A ratios. It was possible to observe that, by the proposed new synthesis method, values of carbonatation efficiency (more than 80%) were obtained, higher than those obtained on W0 sample, or others previously reported in literature [10] [13] . The lowest yield value, c = 10%, was observed for alcoholic samples (W/A = 10%), and they were probably related to fast solvent evaporation (only few minutes) together to the low relative humidity present in the laboratory conditions (R.H. 50%). A high relative humidity value (R.H. 90%) was then chosen to speed up the carbonation process, particularly for the alcoholic nanolime suspension (W/A = 10%). In fact, as reported in literature, carbonation rates were strongly influenced from pH2O; carbonation was sped up at RH ≥ 75% when multilayer adsorption of H2O onto Ca(OH)2 occurred [14] [27] . Measurements of the carbonatation process performed at R.H. 90% were reported in Table 3, for different air exposure times (1, 5 and 7 days). It was possible to note that already after 5 days, the carbonatation process was almost completed (c ≈ 90%), denoting a similar behavior respect to analogous nanoparticles exposed to that humidity conditions [14] [26] .
4. Conclusions
An original, simple and innovative method was proposed, based on the use of an anion exchange resins, having the ability to produce aqueous nanolime suspension with similar if not better features (in terms of size, morphology and reactivity) than current synthetic procedures. In particular, the nanolime particles produced by our method were crystalline, hexagonally plated, and with dimension less than 100 nm. Moreover, if compared with those reported in literature, these particles seemed to be more reactive, reaching values of carbonatation efficiency more than 80% at ambient temperature and for relative humidity conditions of 50%, in particular considering suspensions with water/2-propanol ratios of 100%, 75% and 50% respectively. As concerns the suspension characterized by a water/alcohol ratio of 10%, at high relative humidity conditions (90%), after 7 days the carbonatation process appeared complete, reaching a yield value of 100%.
Furthermore, the proposed method allowed producing nanolime, drastically reducing the time needful for the synthesis (few minutes), working at room temperature and starting from cheap and renewable reactants. Any required intermediate phases (i.e., washings or purification steps) were necessary to eliminate undesired com- pounds, giving the possibility to obtain the same product in a very easy way, with time and costs reductions. Fi- nally, the possibility to regenerate the exhausted resin will allowed reusing it for another synthesis process. This fact, together with simplicity and rapidity of this novel procedure, should provide an ideal opportunity to carry
![]()
Table 2. Estimation of χ values referred to WR2 and W0 suspensions, considering different W/A ratios (T = 20˚C, R.H. = 50%).
![]()
Table 3. Carbonatation process (χ values) referred to WR210 suspension, at different air exposure times (1, 5 and 7 days) and considering a relative humidity condition of 90%.
out a cyclic procedure and so to scale-up Ca(OH)2 nanoparticles production.
NOTES
*Corresponding author.