Characterization of Structure and Property of the Monocarboxyl Bamboo Pulp Fibers ()
1. Introduction
Bamboo pulp fiber is generated cellulose fiber and is prepared by viscose spinning process or Lyocell spinning process with bamboo as raw material, and the bamboo pulp fiber currently used mainly on the domestic market is made by viscose spinning process [1] . Bamboo pulp fiber prepared by this process has favorable dyeability; bamboo pulp fiber fabric has strong moisture absorption and desorption and good permeability; but the breaking strength would decrease after moisture absorption and the shrinkage of bamboo pulp fiber fabric is large, shape retention is poor and fabric wrinkles easily [2] [3] . And partial oxidation of bamboo pulp fiber is an effective way to improve the property of fiber.
Selective oxidation of cellulose is the reaction leads the C2, C3 or C6 hydroxyl in the cellulose macromolecular to aldehyde group, ketone group or carboxyl group. It makes oxidation cellulose own the maternal attributes and can combine with the other substances and increase its uses [4] [5] . Wang Juhua et al. used sodium periodate to oxidize C2, C3 hydroxyl in bamboo pulp fiber and characterized the performance of oxidized bamboo pulp fibe [6] . But reports about the use of selective oxidation of bamboo pulp fiber by HNO3/H3PO4- NaNO2 system [7] are rarely.
In this study, we oxidized the bamboo pulp fiber yarns selectively by HNO3/H3PO4-NaNO2 system and studied the effects of oxidation concentration and reaction time on the structure and property of fiber, so as to provide the basis for the functional modification on bamboo pulp fiber.
2. Materials and Methods
2.1. Materials
Bamboo pulp fiber yarns (28 tex); HNO3 (68% w/v), H3PO4 (85% w/v), NaNO2, ethanol, HCl, calcium acetate and NaOH used for the following investigations were analytical grade.
2.2. Methods
2.2.1. Preparation of Oxidized Bamboo Pulp Fiber Yarns
Nitric acid and phosphoric acid were mixed in 2:1 (v/v) ratio. According to the liquor ratio 1:30, a certain quality bamboo pulp fiber yarn was added to the solution of acid mixture. Once the yarns was completely soaked, a certain mass sodium nitrite were added all at once, the concentration of sodium nitrite were 0.6%, 1.0%, 1.4% and 1.8% (W/V) respectively. The reaction mixture was allowed to react at room temperature, under dark condition and with occasional stirring for 30, 60, 120, 180 or 300 min. Once the oxidation reaction completed, the bamboo pulp fiber yarns were washed with deionized water until the lotion showed a pH of about 4. Then the yarn was soaked in a certain concentration of ethanol for 20 min. Finally it was washed with deionized water and then dried at 60˚C.
2.2.2. Determination of Carboxyl Content
It was performanced according to the method described in the United States Pharmacopoeia [8] . Approximately 0.5 g of the sample, cut into small pieces, was accurately weighed and vibrated in 50 ml of a 2% (w/w) calcium acetate solution for 30 min with ultrasonic oscillator. The mixture was titrated with 0.1 mol/L NaOH using phenolphthalein as an indicator.
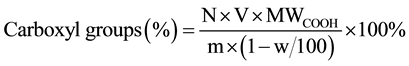
where N is the normality of NaOH, V is the volume of NaOH solution in ml consumed in titration and after correcting for the blank, m is the quality of yarns, and w is the moisture regain of bamboo pulp fiber.
2.2.3. Determination of Weight Loss
The weight of original bamboo pulp fiber yarns was m1, the weight of oxidized bamboo pulp fiber yarns is m2.
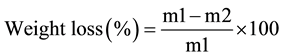
2.2.4. Fourier-Transform Infrared Spectroscopy
The FT-IR spectra were recorded on a Nicolette is 50 FT-IR spectrophotometer using the traditional transmission technique for KBr pellets. KBr pellets were prepared by mixing 1 - 2 mg of the bamboo pulp fiber powders with 200 mg of KBr in a ball mixer and then pelleted.
2.2.5. Scanning Electron Microscopy (SEM)
The surface of varying degrees of oxidized bamboo pulp fibers were observed on a Sirion 200 scanning electron microscopy.
2.2.6. XRD Patterns
XRD patterns of bamboo pulp fiber powders were determined by a model MXPAHF X-ray diffraction system at voltage of 40 kv, current of 30 mA, scan rate of 4˚/min and measurements over 5˚ - 50˚ 2θ.
2.2.7. Determination of Breaking Strength
The breaking strength of bamboo pulp fiber yarns were determined at an effective gauge length of 200 mm and extension rate of 500 mm/min performed on a YG021-A electron single yarn strength tester.
3. Results and Discussion
3.1. Carboxyl Content and Weight Loss of Oxidized Bamboo Pulp Fiber Yarns
The oxidation degree of bamboo pulp fiber yarns were characterized by carboxyl content and results shows in Table 1. The carboxyl contents of bamboo pulp fiber yarns presented in Table 1 were 0.37% and 1.09% respectively after being oxidized 60 min and 120 min in the solution of acid mixture with the concentration of 0.6% NaNO2, were close to the carboxyl contents of bamboo pulp fiber yarns, oxidized for 30 min in the solution of acid mixture with the concentration of 1.0% and 1.4% NaNO2. It indicates that with the extension of reaction time, the carboxyl content of oxidized bamboo pulp yarns increased. To obtain the same carboxyl content of oxidized bamboo pulp fiber yarns, the higher oxidation concentration needed the less reaction time.
Figure 1 shows the weight loss of bamboo pulp fiber yarns in different oxidation condition. As Figure 1 shows, with the increasing of oxidation concentration and extension of reaction time, the weight loss of bamboo pulp fiber yarns increased. When the reaction time is more than 120 min, the weight loss of bamboo pulp fiber yarns increased obviously.
It is plausible that during reaction the C6 hydroxyl of bamboo pulp fiber was oxidized to be carboxyl by HNO3/H3PO4-NaNO2 system but the end units of the cellulose chains were hydrolyzed. Therefore, the weight of bamboo pulp fiber yarns decreased. The oxidation reaction, using HNO3/H3PO4-NaNO2 system as the solution, occurred gradually from amorphous region to crystalline region [9] . In the early stage of oxidation reaction, the end units of the cellulose chains in the amorphous regions are more susceptible to hydrolysis, which caused the weight loss of bamboo pulp fibers. With the extension of reaction time, the oxidation gases spread to the amorphous regions, the hydrogen bond and van der waals between the cellulose chains were decreased or destroyed, the structure of crystalline regions were damaged and the hydrolysis of the end units of the cellulose chains increased, which lead to the dissolution loss of weight. Therefore, the weight of bamboo pulp fiber yarns decreased more seriously. Also with the increase of oxidation concentration, the effects of diffusion and penetration of oxidation gases NO2 and NO to the interior of bamboo pulp fibers and the accessibility of oxidant to the C6 hydroxyl increased. Therefore, with the increase of oxidation concentration, the carboxyl content of bamboo pulp fiber yarns increased but the weight decreased.
3.2. FT-IR Spectra Analysis
Figure 2 shows the FT-IR spectra of the pure bamboo pulp fibers and the oxidized fibers with different carboxyl contents. Cure b, c, d and e are the spectrum cures of oxidized bamboo pulp fibers. Compared with cure a, the spectrum cure of the pure bamboo pulp fibers, a new characteristic absorption band appeared at around 1731.7 cm−1, 1733.6 cm−1, 1735.6 cm−1 and 1741.4 cm−1 respectively due to the stretching vibration of the C=O double bond [10] . And with the increase of oxidation degree, the carboxyl characteristic peaks become higher. This indicates that the C6 hydroxyl were oxidized to be carboxyl partly by HNO3/H3PO4-NaNO2 system.
![]()
Table 1. Carboxyl contents in different oxidation conditions (%).
![]()
Figure 1. Weight loss of oxidized bamboo pulp fiber yarns: (1) NaNO2, 0.6%, (2) NaNO2, 1.0%, (3) NaNO2, 1.4%, (4) NaNO2, 1.8%.
![]()
Figure 2. FT-IR spectra of (a) pure bamboo pulp fiber, (b) bamboo pulp fiber with 0.32% carboxyl content, (c) bamboo pulp fiber with 2.22% carboxyl content, (d) bamboo pulp fiber with 3.58% carboxyl content, (e) bamboo pulp fiber with 8.19% carboxyl content.
3.3. Scanning Electron Microscopic Analysis
The surface of the bamboo pulp fibers before and after oxidizing treatment were observed by SEM. The surface of the pure bamboo pulp fiber shown in Figure 3(a) is smooth and there are many shade stripes. In Figures 3(b)-(e), attachments are found on the surface and with the increase of carboxyl content, twists are found, stripes become less apparent and even disappeared. It illustrates that the surface of bamboo pulp fibers were eroded by the HNO3/H3PO4-NaNO2 system and bamboo pulp fibers contracted.
3.4. XRD Diffraction Analysis
Figure 4 shows the XRD cures of the bamboo pulp fibers before and after oxidation. The peaks of cure a, b, c and d are located near 2θ = 12.5˚, 20.2˚ and 22.2˚, which were the characteristic diffraction peaks of the cellulose II [11] . This indicates that the crystalline structure of bamboo pulp fiber was not altered.
Meanwhile, the crystalline degree of bamboo pulp fiber with 0.32% carboxyl content increased slightly. This is because under low oxidation degree, the end units of the cellulose chains in amorphous zone were mainly eroded away and the rate of amorphous zone decreased. However, with higher oxidation degree, the crystalline degree of bamboo pulp fibers decreased. This can be explained in this way, with the increase of oxidation degree,
![]()
Figure 4. Powder X-ray diffraction patterns of (a) pure bamboo pulp fiber, (b) bamboo pulp fiber with 0.32% carboxyl content, (c) bamboo pulp fiber with 2.22% carboxyl content, (d) bamboo pulp fiber with 3.58% carboxyl content, (e) bamboo pulp fiber with 8.19% carboxyl content.
oxidant spread to the amorphous zone and the force between cellulose chains were destroyed, the hydrolysis of the end units of the cellulose chains increased, the structure of crystalline regions was damaged and the crystalline degree of bamboo pulp fibers decreased. When the carboxyl content increased to 8.19%, diffraction peak near 20.2˚ disappeared and diffraction peak near 22.2˚ become weaker and broader. It is possible that with the increase of oxidation degree, the hydrogen bonds between cellulose chains were destroyed, most of crystalline regions of bamboo pulp fibers transformed into amorphous region and the structure of crystallinity was damaged seriously.
3.5. The Breaking Strength Test
Figure 5 shows the breaking strength of bamboo pulp fiber yarns oxidized by different NaNO2 concentration of
![]()
Figure 5. Breaking strength of oxidized bamboo pulp fiber yarns: (1) NaNO2, 0.6%, (2) NaNO2, 1.0%, (3) NaNO2, 1.4%, (4) NaNO2, 1.8%.
acid mixture. With the increase of oxidation concentration or reaction time, the breaking strength of bamboo pulp fiber yarns decreased. The breaking strength of bamboo pulp fiber yarns oxidized by the solution, whose concentration of NaNO2 was lower than 1.0%, for less than 120 min, could keep more than 75% compared with the breaking strength of pure bamboo pulp fiber yarns. However, the breaking strength was damaged obviously once the oxidation concentration was higher than 1.0% or the reaction time longer than 120 min. The yarns become hard and brittle and the breaking strength could not be tested even when the oxidation concentration increased to 1.8% and reaction time exceeded 180 min.
The breaking strength of yarns were damaged slightly is plausible that in the early stage of oxidation reaction, oxidant could permeate the amorphous regions quickly but had no apparent influence on the crystalline regions and low concentration of oxidant would bring few hydrolysis of the end units of the cellulose chains. As the oxidation reaction progressed, the oxidant spread to the crystalline regions, the disruption of hydrogen bonds between cellulose chains and the hydrolysis of the end units of the cellulose chains progressively increased, causes a linear decrease in the degree of crytallinity and a serious damage in the breaking strength.
4. Conclusions
1) Increasing the oxidation concentration of acid mixture or reaction time could raise the carboxyl content of oxidized bamboo pulp fiber yarns but cause the increase of weight loss. The weight loss of bamboo pulp fiber yarns increased obviously once the reaction time exceeded 120 min.
2) The breaking strength of oxidized bamboo pulp fiber yarns under the condition that oxidation concentration was lower than 1.0% and reaction time less than 120 min could maintain more than 75% compared with the breaking strength of pure bamboo pulp fiber yarns. However, the breaking strength was damaged obviously once the oxidation concentration was higher than 1.0% or the reaction time was longer than 120 min. The breaking strength could not be tested when the oxidation concentration increased to 1.8% and reaction time exceeded 180 min.
3) FT-IR spectra indicated that carboxyl generated in the macromolecule of bamboo pulp fibers during oxidation process. The crystalline degree increased slightly under low oxidation degree but with the increase of oxidation degree, the crystalline degree decreased gradually.