On an Expression of Extraction Constants without the Interfacial Equilibrium-Potential Differences for the Extraction of Univalent and Divalent Metal Picrates by Crown Ethers into 1,2-Dichloroethane and Nitrobenzene ()
1. Introduction
Univalent and divalent metal picrates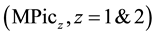 , such as alkali and alkaline-earth metal ones, have
, such as alkali and alkaline-earth metal ones, have
been extracted by crown compounds (L) into the high-polar diluents, such as 1,2-dichloethane (DCE), dichloromethane and nitrobenzene (NB) [1] -[5] . In such high-polar diluents, an extracted ion-pair complex, MLPicz, dissociates MLz+ and zPic− [1] -[3] [6] . In introducing these component equilibria in an extraction model, an individual distribution constant (KD,A) of Pic− (=A−) into the diluents has been determined extraction-experimen- tally [1] -[3] [7] . However, in spite of the limitation of the same KD,A definition and the same diluents, the thus-determined KD,Pic values have differed from each other. For example, the logKD,Pic values were −0.94 [2] for the PbPic2 extraction with 18-crown-6 ether (18C6), −1.34 [7] for the SrPic2 one with benzo-18C6 (B18C6) into NB, −2.46 [3] for the AgPic one with benzo-15-crown-5 ether, −1.89 [2] for the PbPic2 one with 18C6 and −4.35 [6] for the CdPic2 one with 18C6 into DCE. Thus, their values have changed over experimental errors with combinations of MPicz and L.
To clarify a reason for such differences, the authors have applied the idea [8] of an interfacial potential dif-
ference (Dfeq) at extraction equilibrium to an expression of log KD,A, namely 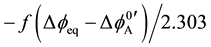 [3] [6]
[3] [6]
[7] , where the negative sign being in the front of f, which denotes F/RT, comes from the electrical charge of A−. In addition to this, extraction constants, Kex± and Kex2±, have been electrochemically expressed as
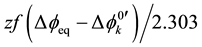 at
at 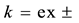 and
and 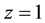 and at ex±, ex2± and 2 [3] [7] . Here,
and at ex±, ex2± and 2 [3] [7] . Here, 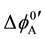 and
and 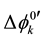 refer
refer
to standard formal potentials for the single distribution of A− into the diluent or organic (o or org) phase and the formal potentials for the overall equilibrium, respectively. Also, Kex± and Kex2± have been defined experimentally
by extraction as 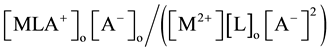 [2] [7] or
[2] [7] or 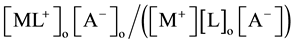 [1] -[3] and
[1] -[3] and 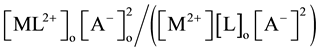 [7] , respectively.
[7] , respectively.
On the other hand, from the thermodynamic points of view, these extraction constants are resolved into 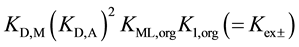 for
for 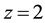 [7] ,
[7] , 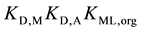 for 1 [3] and
for 1 [3] and
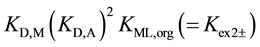 for
for  [7] . Here, the component equilibrium constants, KML,org (complex
[7] . Here, the component equilibrium constants, KML,org (complex
formation in the o phase) and K1,org (1st-step ion-pair formation in the o one), do not contain the Dfeq terms in their expressions, because the constants are of homogeneous systems that all species relevant to the reaction are present in the single o phase [3] [7] ; namely no interface is involved in these processes. Similarly, the distribution constant of Mz+ has been expressed with KD,M (see Equation (3) at  in the Section 2.1) [3] . Therefore,
in the Section 2.1) [3] . Therefore,
since KD,M and KD,A are present in the ![]() or
or ![]() term, the both terms must cancel out mu-
term, the both terms must cancel out mu-
tually the Dfeq ones. Thereby, the extraction constants virtually lose the Dfeq terms on their functional expres-
sions. Thus, the above expression, such as![]() , has caused contradictions on the thermodynamic cycles [3] [7] . Furthermore, such contradictions can cause discrepancies in
, has caused contradictions on the thermodynamic cycles [3] [7] . Furthermore, such contradictions can cause discrepancies in ![]() between
between
experimentally-evaluated values and theoretically-reproduced ones [7] .
In the present paper, in order to solve the above two contradictions, namely the differences of KD,A caused by experimental conditions of extraction and the contradiction based on the thermodynamic cycles [3] [7] , we proposed another expression without Dfeq of the extraction constants, Kex± and Kex2±. In course of clarifying this expression, some experimentally-determined constants [3] [7] , such as Kex±, an individual distribution constant (KD,ML) of the complex ion ML2+ into the NB phase and that of AgL+ into DCE, were also reproduced by calculation. Here, the AgPic and MPic2 (M = Ca, Sr & Ba) extraction with L = 18C6 and/or B18C6 [3] [7] were employed as model systems. Also, a meaning of the Dfeq values [3] [7] & [8] which were calculated from the logKD,A ones determined by the extraction experiments was discussed based on an electroneutrality-point of view [8] for the o phases. Moreover, the thus-obtained expressions for the extraction constants were applied to other types of extraction systems with o = DCE and NB.
2. Theory
2.1. Dfeq Values Derived from Charge Balance Equations for the o Phase
(i) Case of the M(I) extraction with L. For the extraction equilibrium, ![]() , we can obtain from the extraction model (see Appendix I for more details) reported before the following charge-balance equation
, we can obtain from the extraction model (see Appendix I for more details) reported before the following charge-balance equation
![]() (1)
(1)
for the o phase. The concentrations of M+ and A− in the o phase were modified as
![]() (2)
(2)
by using electrochemical equations [6] [8] such as
![]() (3)
(3)
and
![]() (4)
(4)
see Appendix B in ref [6] for a detailed derivation from electrochemical potentials to this equation. Here, ![]() and [j]o/[j] denote a standard formal potential of species j {=M(I), A(−I) & ML(I); see the introduction and section 3.3} and the individual distribution constant (KD,j) of j between the two phases, respectively. At least, the
and [j]o/[j] denote a standard formal potential of species j {=M(I), A(−I) & ML(I); see the introduction and section 3.3} and the individual distribution constant (KD,j) of j between the two phases, respectively. At least, the ![]() values are available from references for M = Ag(I) [9] , Ca(II) [10] , Sr(II) [10] and Ba(II) [10] and A = Pic(−I) [11] into the DCE and NB phases. Additionally, the
values are available from references for M = Ag(I) [9] , Ca(II) [10] , Sr(II) [10] and Ba(II) [10] and A = Pic(−I) [11] into the DCE and NB phases. Additionally, the
![]() values have been determined extraction-experi- mentally [1] -[3] [6] [7] ; see Appendix II for the KD,A determination. Defining as
values have been determined extraction-experi- mentally [1] -[3] [6] [7] ; see Appendix II for the KD,A determination. Defining as ![]() and then rearranging Equation (2), we can easily obtain
and then rearranging Equation (2), we can easily obtain
![]() (5)
(5)
with
![]() (5a)
(5a)
![]() (5b)
(5b)
and
![]() (5c)
(5c)
Accordingly, the following equation is derived.
![]() (6)
(6)
Hence, if the [M+], [ML+]o and [A−] values are determined experimentally, then we can obtain the Dfeq values from Equation (6) immediately; the [ML+]o values were calculated here from the relation
![]() with
with ![]() (see Appendix II for more detail) and
(see Appendix II for more detail) and![]() . The data of [ML+]o £ 0 were neglected in a further computation.
. The data of [ML+]o £ 0 were neglected in a further computation.
(ii) Case of the M(II) extraction with L. Similarly, we can consider the following stepwise extraction-equili- bria [6] [12] at the same time: ![]() (see Appendix I for a basic extraction model and Appendix II for the KD,A determination). Therefore, the charge balance equation for the o phase becomes
(see Appendix I for a basic extraction model and Appendix II for the KD,A determination). Therefore, the charge balance equation for the o phase becomes
![]() (7)
(7)
As described above, this equation was modified to [8]
![]() (8)
(8)
Defining as ![]() and then rearranging Equation (8), we easily obtain the cubic equation
and then rearranging Equation (8), we easily obtain the cubic equation
![]() (9)
(9)
with
![]() (9a)
(9a)
![]() (9b)
(9b)
and
![]() (9c)
(9c)
We can exactly solve this equation for x based on the mathematical formula [13] . Its real solution is
![]() (10)
(10)
where ![]() and
and![]() . Therefore, we can similarly obtain the Dfeq value from the combination of Equations (6) and (10).
. Therefore, we can similarly obtain the Dfeq value from the combination of Equations (6) and (10).
The b¢ values were evaluated from the relation, ![]() with
with ![]() and 2, where
and 2, where ![]() (
(![]() under the condition of
under the condition of ![]() [7] ). The
[7] ). The ![]() values were directly determined by AAS measurements in the
values were directly determined by AAS measurements in the
extraction experiments [2] [7] and also we were able to calculate the other values in r+ from the experimental data [7] .
2.2. On Expressions of the Extraction Constants without Dfeq
According to previous papers, the two of the three extraction constants have been defined as ![]() for the MIA-L extraction system [3] and
for the MIA-L extraction system [3] and ![]() and
and ![]() for the MIIA2-L extraction one [7] . Here, logKex± (or logKex2±) equals
for the MIIA2-L extraction one [7] . Here, logKex± (or logKex2±) equals ![]() (or
(or![]() ) at
) at![]() .
.
These two kinds of extraction constants contain the Dfeq terms as parameters in their functional expressions[3]
[7] . On the other hand, logKex has been expressed as ![]() or
or ![]() without Dfeq and spontaneously became an expression electrochemically-standardized at
without Dfeq and spontaneously became an expression electrochemically-standardized at ![]() [3] [7] .
[3] [7] .
In the above functions, some contradictions have been observed in the former cases: see Appendix in ref. [7] . As an example similar to that described in the introduction, the relation,
![]() , must give a function without Dfeq, because
, must give a function without Dfeq, because
the resulting component equilibrium-constant K2,org does not relate with Dfeq [7] ; namely K2,org and Kex are the constants at![]() . However, using the above definition [3] [7] , the same term,
. However, using the above definition [3] [7] , the same term, ![]() , be-
, be-
comes ![]() and then the Dfeq term does not disappear, where
and then the Dfeq term does not disappear, where ![]() and
and![]() . The same is also true of the result of
. The same is also true of the result of ![]() which is defined as
which is defined as![]() . These two facts obviously have the contradiction with respect to Dfeq.
. These two facts obviously have the contradiction with respect to Dfeq.
In order to cancel such contradictions, we assume here that the two extraction constants are functions without Dfeq, as well as that of Kex [3] [7] . Accordingly, the constants are defined as
![]() (11)
(11)
and
![]() (12)
(12)
That is, by our traditional sense, it is proposed here that complicated equilibrium constants, such as Kex, Kex± and Kex2±, do not contain the Dfeq terms in their functions. This means that these constants are ordinarily defined without Dfeqor under the condition of ![]() and thereby are electrochemically-standardized as
and thereby are electrochemically-standardized as ![]() and
and ![]() [3] [7] . Table 1 lists new (or traditional) expressions of such extraction constants composed of some component equilibrium constants based on thermodynamic cycles.
[3] [7] . Table 1 lists new (or traditional) expressions of such extraction constants composed of some component equilibrium constants based on thermodynamic cycles.
The relations in Table 1 shows that the individual distribution process of A− [12] cancels out that of a cation [14] , such as M+, R4N+, M2+ and ML2+, in Dfeq. As an example, the thermodynamic relation for M(II)
![]() (13)
(13)
can be rearranged into
![]() (14)
(14)
![]()
Table 1. Relations between Kex± or Kex2± and its component equilibrium constants and their corresponding ![]() valuesa.
valuesa.
ak = ex±, ex2±, ML,org, ML,w, & 1,org, where the symbol “w” shows a water phase; bThermodynamic cycle; cRef. [3] ; dRef. [7] .
Therefore, the relation (c) in Table 1 is immediately obtained. From Equations (2) and (8), one should obviously see that Dfeq of KD,M equals that of KD,A in the extraction system of Equation (13). Also, we can rewrite Equation (13) to
![]() (13a)
(13a)
Consequently, Equation (14) or (13) does not contain the Dfeq term and is virtually expressed with only the standard formal potentials (at![]() ) as Equation (13a). The thermodynamic relations are also satisfied with the expressions such as Equations (11) and (12). The same is true of the other relations in Table 1.
) as Equation (13a). The thermodynamic relations are also satisfied with the expressions such as Equations (11) and (12). The same is true of the other relations in Table 1.
3. Results and Discussion
3.1. On a Meaning of Dfeq Estimated from log KD,A
Table 2(a) lists fundamental data [3] for the extraction of AgPic by B18C6 into DCE. The Dfeq values were calculated from Equation (4) and the experimental log KD,Pic values in Table 2(a).
Here, ![]() [11] at 298 K was employed in the calculation.
[11] at 298 K was employed in the calculation.
aValues calculated from ![]() at 298 K; bValues calculated from
at 298 K; bValues calculated from![]() ; cUnit: mol dm−3; dRef. [3] ; eValues re-calculated from the same data as that reported before. See ref. [3] ; fAdditionally determined values which were calculated from the same data as that reported before. See ref. [3] ; gData obtained from additional extraction experiments. Experimental conditions and data analyses are essentially the same as those reported on ref. [3] . For only the data no. 2, the w phases were prepared with about 0.1 mol dm−3 HNO3.
; cUnit: mol dm−3; dRef. [3] ; eValues re-calculated from the same data as that reported before. See ref. [3] ; fAdditionally determined values which were calculated from the same data as that reported before. See ref. [3] ; gData obtained from additional extraction experiments. Experimental conditions and data analyses are essentially the same as those reported on ref. [3] . For only the data no. 2, the w phases were prepared with about 0.1 mol dm−3 HNO3.
*![]() .
.
*![]() §
§![]() .
.
Also, we estimated Dfeq,av from Equation (6) with Equation (5), where Dfeq,av denotes an average value for each run.
The both values, expressed as ![]() &
& ![]() in Table 2(b), agreed well within experimental errors.
in Table 2(b), agreed well within experimental errors.
Average I values of the extraction systems in Table 2(a) were 0.0036 mol×dm−3 for the no. 1A [3] , 0.0028 for 1B, 0.0027 for 1C and 0.097 for 2; I denotes the ionic strength of the water phase in the extraction. Except for the data no. 2, we can handle other three data on the average, because experimental conditions [3] of the data are essentially the same (see the footnote g in Table 2(a) for no. 2). So the following values were obtained at 298 K and L = B18C6: logKex± = 0.31 ± 0.14 and logKD,Pic = −2.54 ± 0.07;
![]() in the IDCE range of (0.40 - 1.1) ´ 10-5 mol×dm−3 (see the data in Table 2(a)) and
in the IDCE range of (0.40 - 1.1) ´ 10-5 mol×dm−3 (see the data in Table 2(a)) and ![]()
in the same IDCE range. The symbol, IDCE, refers to the average ionic strength of the DCE phase; the same is true of INB (see Table 3).
Table 3(a) summarizes the fundamental data [7] for the extraction of MPic2 (M = Ca, Sr & Ba) by 18C6 and B18C6 into NB.
The Dfeq values were calculated from Equation (4) with the logKD,Pic values in Table 3(a) and the
![]() [11] ones reported previously. From Equation (6) with Equation (10), the
[11] ones reported previously. From Equation (6) with Equation (10), the
Dfeq,av values were estimated in the same manner. The above findings are listed in Table 3(b).
For the 18C6 extraction systems, the ![]() values obtained from Equation (4) are close to the
values obtained from Equation (4) are close to the ![]() ones from Equation (6) with Equation (10). On the other hand, the former values are larger than the latter ones for the B18C6 extraction systems.
ones from Equation (6) with Equation (10). On the other hand, the former values are larger than the latter ones for the B18C6 extraction systems.
Except for the ![]() and
and ![]() values of the B18C6 systems, the above results indicate that the interfa-
values of the B18C6 systems, the above results indicate that the interfa-
cial equilibrium-potential differences, Dfeq, based on Equation (4) are essentially the same as those based on
Equation (6). The differences between ![]() and
and ![]() for the B18C6 systems can be due to those in the
for the B18C6 systems can be due to those in the
charge balance equation between extraction experiments (see Appendix II) and electrochemical (or theoretical)
treatments, namely ![]() [7] and Equation (7) or (8). In other words, the condition of
[7] and Equation (7) or (8). In other words, the condition of ![]() cannot be satisfied in the B18C6 systems. For example, an average value of
cannot be satisfied in the B18C6 systems. For example, an average value of ![]() was 0.12 for L = B18C6, while that was 0.029 for 18C6; these values
was 0.12 for L = B18C6, while that was 0.029 for 18C6; these values
were the maximum of the B18C6- and 18C6-M(II) extraction systems. Practically, the ![]() values based on Equation (7) or (8) must be more accurate than the
values based on Equation (7) or (8) must be more accurate than the ![]() ones.
ones.
On the basis of the above facts, ![]() and
and![]() , we see that the Dfeq value obtained from the distribution process of
, we see that the Dfeq value obtained from the distribution process of ![]() is essentially equivalent to that from the combined process of
is essentially equivalent to that from the combined process of ![]() and
and ![]() [8] {see Equations (1) & (2)} or
[8] {see Equations (1) & (2)} or![]() ,
, ![]() and
and ![]() {see Equations (7) & (8)} into
{see Equations (7) & (8)} into ![]() and NB.
and NB.
3.2. Experimental Proof of Kex± and Kex2± without Dfeq
We obtained the log Kex± values of the AgPic extraction with B18C6 into DCE from the relation (a) in Table 1 with ![]() [9] (
[9] (![]() [3] ),
[3] ), ![]() [11] (into DCE) and the corres
[11] (into DCE) and the corres
ponding logKML,DCE value in Table 2(a). These values, expressed as ![]() below, are in good agreement with those listed in Table 2(a).
below, are in good agreement with those listed in Table 2(a).
The KD,AgL calculation can be an indirect proof of Kex± without Dfeq. First, the log KD,AgL values (namely ![]() ones) standardized at
ones) standardized at ![]() for L = B18C6 were calculated from the modified form,
for L = B18C6 were calculated from the modified form, ![]() , of the relation (b) in Table 1. The obtained values are shown as
, of the relation (b) in Table 1. The obtained values are shown as ![]() in Table 2(d). In this calculation, we employed
in Table 2(d). In this calculation, we employed ![]() [11] (into DCE),
[11] (into DCE), ![]() [15] (in water),
[15] (in water), ![]() [16] at 298 K.
[16] at 298 K.
Next, the logKD,AgL values were reproduced by using the equation,
![]() at 298 K (see Appendix in ref. [3] for its detailed derivation), with the calculated
at 298 K (see Appendix in ref. [3] for its detailed derivation), with the calculated ![]() values and the
values and the ![]() ones. These
ones. These ![]() values in
values in
Table 2(d) are in good accordance with the values listed in Table 2(a). Thus the log KD,AgL values can be well
reproduced. From the results of ![]() &
& ![]() at least, we can see that Equation (11) is valid for the Ag
at least, we can see that Equation (11) is valid for the Ag
Pic-B18C6 extraction system.
Moreover, an average ![]() value for all the
value for all the ![]() ones was 1.39 ± 0.23. From this value and the
ones was 1.39 ± 0.23. From this value and the ![]() ones, we calculated the logKD,AgL values again, using the above relation [3] . The value obtained from
ones, we calculated the logKD,AgL values again, using the above relation [3] . The value obtained from ![]() of no. 1C was under-estimated by½0.3½ and that of no. 2 was over-estimated by the same, compared to those in Table 2(a) or of
of no. 1C was under-estimated by½0.3½ and that of no. 2 was over-estimated by the same, compared to those in Table 2(a) or of![]() . On the other hand, the logKD,AgL values (= 3.1 & 2.7, respectively) of nos.
. On the other hand, the logKD,AgL values (= 3.1 & 2.7, respectively) of nos.
1A and 1B were close to those in Table 2(a).
The logKex± values for the M(II)-B18C6 extraction into NB were calculated from the relation (c) in Table 1.
These ![]() values are in accordance with the values in Table 3(a); the logKex± values in Table 3(a) have
values are in accordance with the values in Table 3(a); the logKex± values in Table 3(a) have
been determined by the procedure [2] [7] described in Appendix II. This accordance indicates that Equation (11)
without Dfeq is satisfied. In this calculation, ![]() ,
, ![]() ,
, ![]()
[10] , logKCaL,NB = 11.2, logKSrL,NB = 13.1, logKBaL,NB = 13.4 for L = 18C6 [17] , logKCaL,NB = 9.43, logKSrL,NB = 11.1 and logKBaL,NB = 11.6 for L = B18C6 [17] were employed. Also, the logKD,M values were calculated from
the modified form of Equation (3), ![]() , with the
, with the ![]() values, where the
values, where the ![]() values in Table 3(a) corresponding to them were employed accordingly.
values in Table 3(a) corresponding to them were employed accordingly.
The following discussion is similar to that from ![]() to KD,AgL at L = B18C6 (Table 2(d)). The
to KD,AgL at L = B18C6 (Table 2(d)). The ![]() values at M(II) were calculated from a modified form,
values at M(II) were calculated from a modified form, ![]() , of the relation (f) in Table 1. Here, the adopted
, of the relation (f) in Table 1. Here, the adopted![]() , in water at 298 K} val-
, in water at 298 K} val-
ues were 0.48 for the Ca-18C6 [18] and -B18C6 [19] systems, 2.72 [20] for Sr-18C6, 3.87 [20] for Ba-18C6, 2.41 [15] for Sr-B18C6 and 2.90 [13] for Ba-B18C6. Also, ![]() [11] (into NB), logKD,18C6 = −1.00 [21] and logKD,B18C6 = 1.57 [17] (into NB) at 298 K were used for calculation. Furthermore, from the assumption in the section 2.2, we employed the logKex2± values [12] which have been reported before and their values virtually correspond to the ones standardized at
[11] (into NB), logKD,18C6 = −1.00 [21] and logKD,B18C6 = 1.57 [17] (into NB) at 298 K were used for calculation. Furthermore, from the assumption in the section 2.2, we employed the logKex2± values [12] which have been reported before and their values virtually correspond to the ones standardized at ![]() (see Table 3(a)).
(see Table 3(a)).
The calculated ![]() values are listed in Table 3(d). These values agreed well with those [17] previously-reported by the ion-transfer polarographic measurements, except for the Ba-18C6 and -B18C6 systems. This fact indirectly indicates that Equation (12) is satisfied. For the Ba-18C6 and -B18C6 systems, −2.6 for the former and −0.8 for the latter have been reported [17] .
values are listed in Table 3(d). These values agreed well with those [17] previously-reported by the ion-transfer polarographic measurements, except for the Ba-18C6 and -B18C6 systems. This fact indirectly indicates that Equation (12) is satisfied. For the Ba-18C6 and -B18C6 systems, −2.6 for the former and −0.8 for the latter have been reported [17] .
As similar to ![]() in Table 2(d), the calculation of logKD,ML becomes the indirect proof of logKex2±
in Table 2(d), the calculation of logKD,ML becomes the indirect proof of logKex2±
without Dfeq. Then, the logKD,ML values at 298 K were estimated from the ![]() ones and the equation,
ones and the equation, ![]() [7] ; the
[7] ; the ![]() values were used here.
values were used here.
The thus-calculated ![]() values were close to the values listed in Table 3(a); the experimental
values were close to the values listed in Table 3(a); the experimental
logKD,ML values in Table 3(a) have been calculated from the relation (d) in Table 1 [7] . This fact indicates that Equation (12) satisfies indirectly the thermodynamic cycle of (f).
aRef. [7] ; bUnit: mol dm−3; c![]() values: see ref [12] ; dValues re-calculated from the data in ref [12] .
values: see ref [12] ; dValues re-calculated from the data in ref [12] .
*![]()
*![]() . §
. §![]() .
.
The above calculation results for the AgPic and MPic2 extraction with L indicate that the assumption of Equations (11) and (12) without Dfeq is essentially valid. In other words, the overall extraction constants, Kex± and Kex2±, must be expressed rationally as functions without Dfeq.
3.3. For Applications to Other Extraction Systems
The above handling based on Table 1 can be also applied to the practical extraction equilibria of
![]() into o = NB [14] , (E11)
into o = NB [14] , (E11)
![]() into DCE [22] and CH2Cl2 [23] , (E12)
into DCE [22] and CH2Cl2 [23] , (E12)
![]() into IL = an ionic liquid phase [24] [25] , (E13)
into IL = an ionic liquid phase [24] [25] , (E13)
![]() into DCE [26] , (E14)
into DCE [26] , (E14)
![]() into NB [27] (E15)
into NB [27] (E15)
and
![]() into NB [28] . (E16)
into NB [28] . (E16)
As examples, thermodynamic points of view suggest the following cycles for the above equilibria:
![]() (E11c)
(E11c)
![]() (E12c)
(E12c)
![]() (E13c)
(E13c)
![]() (E14c)
(E14c)
with ![]() and
and![]() ,
,
![]() (E15c)
(E15c)
and
![]() (E16c)
(E16c)
with![]() ,
, ![]() and
and![]() , respectively. Similarly, only the KD,j values are expressed as
, respectively. Similarly, only the KD,j values are expressed as
functions with the Dfeq ones.
The relation, ![]() , for the process (E11) can be arranged into
, for the process (E11) can be arranged into![]() . This does not contradict the fact [14] that the determination of
. This does not contradict the fact [14] that the determination of ![]() by solvent extraction experiments gives
by solvent extraction experiments gives ![]() and
and![]() , when either KD,M or KD,A was standardized at
, when either KD,M or KD,A was standardized at ![]() which is based on the Ph4As+BPh4- assumption [14] [29] & [30] . Also, KD,C cancels out KD,A in (E12c):
which is based on the Ph4As+BPh4- assumption [14] [29] & [30] . Also, KD,C cancels out KD,A in (E12c):![]() . For
. For ![]() and
and![]() , the
, the ![]() value becomes 2.66 (
value becomes 2.66 (![]() [22] ) and accordingly we have obtained the
[22] ) and accordingly we have obtained the ![]() value at 298 K from the experimental
value at 298 K from the experimental ![]() one [11] .
one [11] .
Similarly, KD,T cancels out KD,A in (E13c), where T- denotes another anion. That is,
![]() . For the overall
. For the overall
equilibria, (E14) & (E15), one can handle them in the same manner as that described above for the AgPic and MPic2 extraction with L, respectively.
We can easily see that the KD,H and KD,Pu values cancel out the KD,Cl one in (E16c). That is,
![]() equals
equals ![]() and then becomes
and then becomes ![]() . We found the
. We found the ![]() value {
value {![]() = 0.035 V [29] at 298 K}, but were not able to find the
= 0.035 V [29] at 298 K}, but were not able to find the ![]() value in references.
value in references.
4. Conclusion
It was demonstrated that the Dfeq values calculated from the experimental logKD,Pic ones are in agreement with or close to those more-accurately done from the charge balance equations for the species with M(I) in the DCE phase and with M(II) in the NB one, except for some cases. This demonstration indicates that the plots of ![]() versus
versus![]() , described in Appendix II with
, described in Appendix II with ![]() & 2, yield the practical KD,A
& 2, yield the practical KD,A
values and then the first-approximated Dfeq ones. These results will give an answer to how one explain the differences in KD,A among extraction experiments of various MA or MA2 by various L. Also, we clarified that the assumption of Equations (11) and (12) is valid for the AgPic and MPic2 extraction with 18C6 and/or B18C6. This eliminated the contradictions [3] [7] due to Dfeq from the thermodynamic cycles. Moreover, the present work indicates a possibility that the proposed handling can be applied to various extraction systems with neutral ligands at least.
Acknowledgements
The authors thank Mr. Tomohiro Amano, Mr. Satoshi Ikeda and Mr. Yuki Ohsawa for their experimental assistances.
Appendix I
The basic extraction model [1] [3] [31] for the case (i) is as follows.
![]() (corresponding equilibrium constant is KML), (A1)
(corresponding equilibrium constant is KML), (A1)
![]() (K1), (A2)
(K1), (A2)
![]() (KD,MLA), (A3)
(KD,MLA), (A3)
![]() , (A4)
, (A4)
![]() (A5)
(A5)
and
![]() (KMA). (A6)
(KMA). (A6)
Consequently, these component equilibria yield those of ![]() (KD,M),
(KD,M), ![]() (KD,ML),
(KD,ML), ![]() (KD,L) and
(KD,L) and ![]() (KD,A). An extraction of HPic,
(KD,A). An extraction of HPic, ![]() (Kex,HPic), was added in the
(Kex,HPic), was added in the
[Pic-] calculation. The distribution [31] of ![]() into the DCE phase was neglected in this study; its constant was not available from references.
into the DCE phase was neglected in this study; its constant was not available from references.
The case (ii) [2] [6] [7] was
![]() (KML), (A7)
(KML), (A7)
![]() (K1), (A8)
(K1), (A8)
![]() (K2), (A9)
(K2), (A9)
![]() (KD,MLA2), (A10)
(KD,MLA2), (A10)
![]() , (A11)
, (A11)
![]() , (A12)
, (A12)
![]() (A13)
(A13)
and
![]() (KMA+), (A14)
(KMA+), (A14)
where the distribution of ![]() into the NB phase was neglected; their constants were not available from references. Similarly, some equilibria, such as
into the NB phase was neglected; their constants were not available from references. Similarly, some equilibria, such as ![]() (KD,M),
(KD,M), ![]() (KD,ML) and
(KD,ML) and![]() , can be given from the above component equilibria and the Kex,HPic value was included in the calculation.
, can be given from the above component equilibria and the Kex,HPic value was included in the calculation.
The both models, (i) & (ii), do not contain supporting electrolytes in the o phases. This point is a large difference from corresponding electrochemical measurements [29] [30] .
Appendix II
The KD,A values have been determined extraction-experimentally using the following equations [1] -[3] [6] [7] .
![]() (A15)
(A15)
![]() (A16)
(A16)
for ![]() at
at ![]() (the case of M+) or for
(the case of M+) or for ![]() at
at ![]() (that of M2+). Hence, the plots of
(that of M2+). Hence, the plots of ![]()
versus ![]() [1] [3] and versus
[1] [3] and versus ![]() [2] [6] [7] based on Equation (A16)
[2] [6] [7] based on Equation (A16)
give the KD,A value with the Kex ones for the MA- and MA2-L extraction systems, respectively. Here, the
![]() values are determined by AAS measurements and then the [Mz+], [L]o and [A−] values are
values are determined by AAS measurements and then the [Mz+], [L]o and [A−] values are
calculated by a successive approximation [1] -[3] [6] [7] . The following mass-balance equations have been
employed for the approximation: ![]() [1] [3] against Equation (1) and
[1] [3] against Equation (1) and ![]() [2]
[2]
[6] [7] against Equation (7) (see the Section 3.1).
Similarly, the Kex± values have been evaluated from the other arranged form of Equation (A15),
![]() (A17)
(A17)
for ![]() at
at ![]() or for 1, 2 at 2 [3] [7] .
or for 1, 2 at 2 [3] [7] .
NOTES
*Corresponding author.