1. Introduction
Carbon dioxide is one of the major greenhouse gases (GHGs). Starting early in 19th century, extensive anthropogenic CO2 emissions due to fossil fuel utilization have led to carbon dioxide accumulation in the atmosphere. According to the Intergovernmental Panel on Climate Change (IPCC) the increase in greenhouse gases has triggered serious climate changes demonstrated more obviously as an increase in earth temperature. In this context, the development of highly efficient CO2 sorbents for trapping large amounts of CO2 from concentrated sources such as natural gas processing and power plants emission emerges as a pressing issue. Among several available processes for carbon dioxide capture, CO2 capture with amines is a well-established technology [1] -[4] . Still, alternative processes such as effective and less expensive solid CO2 sorbents based on amine immobilization on porous supports are extensively investigated with the goal to develop recyclable solid sorbents to achieve competitive and less energy intensive acid-gas removal alternatives [5] -[15] . Unlike liquid sorbents, solid sorbents yield less waste during cycling. Additionally, the spent solid sorbents can be disposed more easily and in an environmentally friendly way. There still remain several challenges before amine immobilized CO2 sorbents become industrially practical. High-surface-area silica such as MCM-41 [5] , MCM-22, MCM-36 and ITQ-2 [6] and many others [7] have been widely used as supports for immobilizing various types of amines for CO2 capture. In particular, amine functionalized solid sorbents are appealing [8] -[18] because the high capture capacity of the amine molecules is retained [15] [16] . Various other adsorbents such as zeolites [19] [20] , activated carbons [21] -[24] , activated aluminas [25] -[27] , and membranes [28] have also been investigated. Zeolite based sorbents showed high adsorption capacity at lower temperatures, but their capacity declines above room temperature [21] [22] . In addition, since these sorbents also absorb moisture and other gases, their CO2 separation selectivity is low, resulting in large separation systems with high operational cost in practical applications.
Grafting of amines on porous solids is one way to enhance the adsorption capacity of sorbents [28] [30] . Solid-supported amines possess significant advantages such as low energy cost for sorbent regeneration, high selectivity and reversibility towards CO2 and ease of disposal. In addition, solid amine sorbents have been proved to be tolerant towards moisture and, thus, more effective for capturing humidified CO2 compared with zeolites and activated carbons [19] -[24] . Among various solids which could be functionalized with amines for CO2 sorption, Mesoporous silica have been attracted much attention [15] -[17] . This category of porous materials possesses several desirable properties such as large surface area, tuneable pore structure, and high thermal stability. These properties make Mesoporous silica a promising support for amine immobilization. To date a series of amine supported Mesoporous silica have been prepared and investigated [15] -[18] . In general, Mesoporous silica foams with large pore size and pore volume are better supports compared to other mesoporous siliceous materials. Amine functionalized supports such as MCM-41 [5] , MCM-22 and ITQ-2 [6] showed lower CO2 capacities. On the other hand, these amine based sorbents showed better selectivity for CO2 adsorption over N2 or CH4. [15] [16] .
The objective of this work was to investigate the CO2 sorption mechanism on amine functionalized Mesoporous silica and maximize the sorption capacity. A properly designed PEI/MPS sorbent was synthesized for use intheCO2 sorption studies. XPS was employed to follow changes in the surface chemical bonding while CO2 was interacted with the solid sorbent. Lastly, high CO2 pressure adsorption experiments were conducted in an effort to enhance the sorption capacity of the hybrid sorbent.
2. Experimental
2.1. Chemicals
Triblock co-polymer poly (ethylene oxide)-b-(propylene oxide)-b-poly(ethylene oxide) surfactant P123 (EO20PO70- EO20, Mv = 5800), sodium silicate, acetic acid, ammonium fluoride, polyethylenimine, PEI10K, Mn ≈ 10,000), Span 80 surfactant and ethanol (v/v = 90%) were all purchased from Aldrich and used without further purification. Deionized water was used in all experiments.
2.2. Preparation of Foam Support
The Mesoporous silica (MPS) support was prepared as reported previously [15] [16] . Briefly 3.0 g of P123 was dissolved in a solution of acetic acid (3.0 g), water (52 g), and ammonium fluoride (0.3 g) at 40˚C. A solution of sodium silicate (2.35 g) in water (40 g) was heated to 40˚C and poured into the surfactant solution under vigorous stirring. The mixture was kept under static conditions at 40˚C for 24 h and aged at 70˚C for another 24 h. The product was collected by filtration, followed by copiously washing with DI water. The surfactant P123 was removed by calcination at 560˚C for 6 h.
2.3. Sorbent Preparation
PEI-10K (10,000 g/mol) was impregnated into the Mesoporous silica. In a typical batch, a certain amount of PEI was added into 10 mL of dry ethanol. After dissolution, 1 g of Mesoporous silica was added under stirring followed by statically keeping the mixture at room temperature for 12 h. The resultant slurry was dried at 100˚C for 16 h. The obtained sample was denoted as PEI10K-MPS.
2.4. CO2 Sorption/Desorption Performance
The CO2 sorption-desorption of the PEI/MPS sorbents were monitored using a Pyris 6 TGA Perkin Elmer thermal gravimetric analyzer (TGA) by following the weight increases/decreases during the sorption and desorption process in pure CO2. About 10 mg of the sorbent was put into the TGA measuring pan, the temperature was increased from room temperature to 110˚C at a heating rate of 5˚C/min and equilibrated at 110˚C for 30 min under pure N2 (99.99%) at a flow rate of 20 ml/min to remove any moisture or remaining solvent from the sorbents. The temperature was then allowed to drop to the desired sorption temperature and the gas was switched from N2 to pure CO2 (99.9%), held for 90 min for CO2 sorption, and then switched back to N2 for 30 min for CO2 desorption while the temperature was kept fixed. The CO2 sorption capacity (mmol CO2/g sorbent) was calculated from the weight change of the sample measured by TGA during the sorption/ desorption processes. Note that the sorption capacity measured by TGA in this study is an equilibrium sorption capacity, as CO2 was allowed to flow till the weight of the sample reached its maximum value. The high pressure CO2 adsorption capacity was determined using a Rubotherm Magnetic Suspension Balance (MSB). In a typical high pressure CO2 adsorption-desorption isotherm measurement, approximately 50 mg of PEI-10k/MPS was placed on an alumina crucible. The system was taken under vacuum for 3 h at 85˚C. CO2 adsorption isotherms were measured at 85˚C by raising the pressure from ambient to 30 atm.
2.5. Characterization Techniques
Surface areas were calculated by the simplified Broekhoff-de Boer method from N2 sorption experiments. The total pore volume was estimated from the amount of N2adsorbed at a relative pressure of 0.99. The pore size and the size distribution of the foam support and the sorbents were measured by the nano scanning electron microscope (NSEM) (NOVANANOSEM450).
Full elemental surface analysis was carried out by X-ray photoelectron spectroscopy (XPS) along with reflected electron energy loss spectroscopy (REELS). The latter was used to detect and quantify hydrogen. AllXPS and REELS spectra were taken on a Thermo Fisher Scientific spectrometer. Pressure in the analytical chamber during spectral acquisition was less than 5 × 10−9 torr with the surface analysis depth range from 30 - 50 Angstroms. The energy for survey and high resolution scans was 80 and 20 eV, respectively. XPS and REELS analysis were carried out on the bare Mesoporous silica sample and PEI-10k/MPS before and after CO2 adsorption. To investigate the CO2 interaction, the PEI-10k/MPS sorbent was exposed to 1 bar of CO2 inside a catalytic cell that is directly connected to the UHV chamber. The PEI-10k/MPS sorbent was heated at 85˚C prior to admitting CO2 for 30 min. The catalytic cell was then evacuated before transferring the CO2 treated PEI-10k/MPS to the UHV chamber for XPS analysis.
3. Results and Discussion
3.1. Characterization of Sorbents
PEI-10k was chosen for immobilization on the MPS support because of its high thermal stability at the desired temperatures for CO2 capturing (70˚C to 90˚C). Figure 1 shows that MPS-10/MPS sorbent was stable till about 200˚C. This in comparison with the MPS support itself. Below 200˚C the PEI 10k neither evaporated nor decomposed below. The N2 adsorption/desorption isotherms of the MPS support and the hybrid MPS-10/MPS sorbent along with their B.J.H. pore size distributions are shown in Figure 2 and Figure 3, respectively. Both pure MPS and MPS-10/MPS show the classic type IV isotherm. The functionalized sample shows a smaller B.J.H. surface area and narrower pore size distribution compared to the pristine MPS. As observed from Figure 3, PEI-10k/MPS still presents mesopore properties and had available porosity. Indeed, the XPS study below will show that PEI was evenly distributed between the surface and the bulk of the sorbent suggesting that it is not
![]()
Figure 1. Thermogravimetric (TGA) graphs of PEI-10k/MPS and MPS. Mass loss either due to evaporation or decomposition of PEI-10k/MPS did not occur below 200˚C.
![]()
Figure 2. N2 adsorption-desorption isotherms at 77 K measured using Broekhoff-de Boer methodfor (a) MPS and (b) amine functionalized sample.
![]()
Figure 3. Pore size distribution calculated using the Broekhoff-de Boermethod for (a) MPS and (b) PEI-10k/MPS.
only present in the porous of the silica, but surrounding the silica particles as well.
The cumulative surface areas of the MPS foam and the PEI-10k/MPS as calculated using the simplified Broekhoff-de Boer method are 750 and 38 m2∙g−1, respectively, Figure 2. The corresponding cumulative pore volume of the MPS and PEI-10k/MPS foams as also calculated by the Broekhoff-de Boer method are 2.5 and 0.13 cm3∙g−1, respectively, Figure 3. The morphology of the support before and after amine impregnation was investigated by NSEM. Representative SEM images are shown in Figure 4. The average pore size of the MPS is about 60 nm in a good agreement with the pore sizes calculated above, Figure 3. The images in Figure 4 show that a significant amount of PEI molecules is immobilized inside the mesoporous foam cells with some on the external surfaces. Nonetheless, the hierarchical porous structure within and between the sorbent particles is retained facilitating diffusion of CO2 gas in the sorbent.
XPS and HREELS were used to fully quantify the surface elemental composition of the CO2 sorbents. As expected, XPS of the PEI10k/MPS revealed the presence of N, C, O and Si. Surface and bulk elemental concentrations are given in Table 1. The decrease of the Si and O from the surface indicates that the PEI was highly dispersed on the surface. The N and C content are similar on both the surface and in the bulk suggesting that the PEI was evenly distributed between surface and bulk. The hydrogen content was significantly greater on the surface of the PEI/MPS than in the bulk. Since the surface O was still less than in the bulk and the ratio O:Si on the surface and in the bulk were 2.02 and 1.14, respectively, some of the surface oxygen, and consequently, hydrogen must be due to chemisorbed water, which explains the excess of hydrogen on the surface.
The reaction between primary and secondary amines with CO2 leads to the formation of carbamate as presented in Equations 1 and 2, respectively. The chemical reaction mechanism between primary or secondary amino groups with CO2 proceeds via a zwitterion intermediate [31] -[35] . The reaction pathway consists of two steps. First, a zwitterion is formed between the amino group and the carbon dioxide molecule. Then, a base is
![]()
Figure 4. Scanning electron nanographs of (a) MPS and (b) PEI-10k/MPS.
![]()
Table 1. Atomic percent of elemental bulk and surface composition of PEI-10k/MPS sorbent.
(a)The percent of elements was calculated for the immobilized 70% PEI-10k in MPS. (b)Determined by XPS determined except for H, which was quantified by HREELS.
necessary to stabilize the zwitterion and to obtain a carbamate as a final product. If water is present in the media it can act as a base, receiving the proton transferred by the zwitterion. But in this case, as no other base is present, another amino group acquires a positive charge forming the carbamate according to reactions 1 and 2 for primary and secondary amines, respectively.
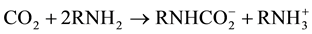 (1)
(1)
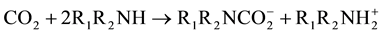 (2)
(2)
For carbamate the end group contains C and N as well as O atomic species, and hence single peak features at 285, 399 and 530.1 eV were measured and analysed in the respective 1s spectra. Figure 5 shows the XPS spectra of PEI 10k/MS before and after CO2 treatment. The detailed C 1s, O 1s and N 1s core-level spectra corresponding to PEI 10k/MPS/CO2 and (top) and PEI 10k/MPS (bottom) are also shown in Figure 6. The nitrogen 1s occurs at a binding energy consistent with a typical amine functional group (399 eV). After CO2 treatment the N 1s peak was broad and could be resolved into two components: a high binding energy (~401 eV) and a lower binding energy one (396 eV). The high binding energy component is consistent with strongly ionized state of nitrogen state, possibly protonated nitrogen atoms of the carbamate species formed in reactions 1 and 2 [36] . On the other hand, the lower binding energy N 1s component (~396 eV) could possibly be due to strongly adsorbed CO2 on any of the nitrogen atoms (primary, secondary or tertiary). Further detailed XPS investigation is planned to fully quantify the contribution of CO2 to the chemically formed carbamate and the strongly adsorbed CO2.
The C 1s spectra of the PEI/MPS occurred at 285.8 eV, which could be due to a combination of C-C and C-N boding. This binding energy value is a little bit higher than that of C-C single bond (which is typically at 285 eV). The high-energy value could be due to the conjugation nature of the C-C bond in the PEI-10k due to the neighbouring nitrogen atoms. When covered with CO2, the C 1s spectra was broad and its maximum intensity was shifted down to 284.8 eV. Lowering the C 1s binding energy after CO2 adsorption strongly supports the conjugation nature of the C-C bond in the PEI molecule as the nitrogen lone pairs were involved in the N-CO2 surface chemical bond at the expense of the conjugation of the C-C and C-N bonds. The shoulder at ~284 eV is possibly due to the N-CO2 surface chemical bonds.
3.2. CO2 Adsorption-Desorption Performance
3.2.1. Influence of Temperature
The effect of temperature on the adsorption process was studied by conducting adsorption experiments at 65˚C
![]()
Figure 5. X-ray photoelectron spectra of PEI-10k/MPS: before CO2 adsorption (lower) and after CO2 adsorption (upper).
![]()
Figure 6. Detailed XPS 1s spectra of N, C and O of PEI-10k/MPS before and after CO2 exposure.
and 75˚C, 85˚C and 95˚C. Adsorption/ desorption plots are shown in Figure 7. As observed, the CO2 adsorption capacity clearly increases with temperature. A large enhancement in the adsorption capacity at 1 bar was noticed, when the temperature was increased from 65˚C to 85˚C. Since the reaction between CO2 and amino groups is exothermic [32] [37] [38] , the observed temperature dependence is explained in terms of kinetic effects rather than thermodynamics. Indeed, the rate constants, as determined in our labs via the double exponential model were greatly increased by changing the temperature from 55˚C to 85˚C. The temperature increase promotes a higher mobility, increasing the kinetics of access of CO2 to the regions that are not easily accessible at lower temperatures. The result of this is the increase of CO2 uptake with temperature. This effect is also produced by faster reaction kinetics between CO2 and the amino groups at higher temperatures. On the other hand, another possible explanation has been proposed to explain similar temperature dependences observed with other PEI- immobilized systems [39] . The increase of CO2 adsorption capacity with temperature has been attributed to a singular expansion of PEI aggregates within the pores of the mesostructured materials, when temperature is increased. According to these observations, at low temperature, PEI resides inside the channels of the support, and only the external active sites of PEI are accessible to CO2 molecules. In contrast, at higher temperatures PEI expands occupying all the available space in the pores, thus becoming more active towards CO2 [32] [39] .
In an attempt to calculate the CO2 capture efficiency of the sorbent, the concentration of the active sites (i.e. the primary and secondary amine groups) were calculated and correlated to the maximum amount of adsorbed
![]()
Figure 7. (a) adsorption and (b) desorption profiles at 1 bar of CO2 flow at various temperatures. The adsorption and dsesorption profiles were taken using TGA measurements.
CO2.
For 1 g of PEI10k, the N content is 0.02325 moles as the molecular weight of the repeat unit of the PEI, CH2CH2NH, is 43. So for the present sorbent PEI10k/MPS with an amine content of 70 wt%, the total N content is 0.01628 mole/g of sorbent. Note that under dry conditions only primary and secondary amines are active for CO2 carbamate formation. Also, according to the PEI manufacturer, the ratio between primary: secondary: tertiary amines is 1:2:1. Moreover, the formation of a carbamate species requires two amines (according to Equations (1) and (2)). Therefore, the maximum theoretical sorption capacity of CO2 on PEI-10k/MPS is 6.1 mmol CO2/g sorbent. So far under ambient pressure, the maximum experimental sorption capacity at 85˚C was close to 4 mmol of CO2/g sorbent, which corresponds to 65% efficiency. A possible approach to enhance the sorption efficiency is by raising the gas pressure as discussed below.
3.2.2. Influence of Pressure
It is well-known that the gas pressure is a crucial factor for the sorption capacity of various sorbents. Hence, PEI10k/MPS was selected to investigate the effect of the CO2 pressure on the sorption capacity using TGA with pure CO2 gas at pressures ranging from 0 to 25 bar. Figure 8 shows CO2 adsorption isotherm obtained for PEI 10k/MPS. In this isotherm, the amount of CO2 adsorbed after reaching equilibration, expressed as mmol CO2/g of adsorbent, is plotted against gas pressure in bar. The CO2 sorption capacity became greater with increasing pressure. At 24.94 bars the capacity was 8.36 mmol CO2/g sorbent. This value is greater than the theoretical efficiency, which was previously calculated as 6.11 mmol CO2/g sorbent. An explanation for this increased efficiency could be due to chemisorbed CO2 in addition to the chemically formed carbamate. Raising the pressure favors chemisorption and can create new sites for CO2 capturing. The unreacted tertiary amine groups could be the active surface sites for the chemisorbed CO2. Every tertiary amine group could act as a possible site for chemisorbed adsorption. Therefore, about 4.07 mmole of CO2/g sorbent could still be adsorbed before reaching saturation.
The plots show that, as expected, mads increases non-linearly with the CO2 partial pressure, 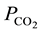 ,
,
The obtained experimental values of mads were fitted to the Langmuir equation, according to:
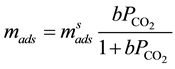 (3)
(3)
where 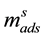 denotes the moles adsorbed until saturation and b the affinity coefficient between the adsorbent and adsorbed phases. The affinity coefficient b (Pa−1) is a function of heat of adsorption
denotes the moles adsorbed until saturation and b the affinity coefficient between the adsorbent and adsorbed phases. The affinity coefficient b (Pa−1) is a function of heat of adsorption 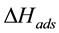 (kJ/kg,), and since it is an exothermal process it a has negative value for CO2 adsorption. The affinity coefficient, b, is given by the relation:
(kJ/kg,), and since it is an exothermal process it a has negative value for CO2 adsorption. The affinity coefficient, b, is given by the relation:
 (4)
(4)
![]()
Figure 8. Measured adsorption isotherm measured at 85˚C (red) and fitted with a Langmuir adsorption relation (black).
![]() (Pa−1) in Equation (4) is a constant and R is the gas constant. According to the Equations (3) and (4), the CO2 adsorption capacity should decrease as the adsorption temperature increases, due to the exothermal nature of the adsorption process. This really happened at temperatures above 85˚C, Figure 7(a). In this case the thermodynamic effect prevailed the kinetic factors, which were more pronounced at temperatures below 85˚C
(Pa−1) in Equation (4) is a constant and R is the gas constant. According to the Equations (3) and (4), the CO2 adsorption capacity should decrease as the adsorption temperature increases, due to the exothermal nature of the adsorption process. This really happened at temperatures above 85˚C, Figure 7(a). In this case the thermodynamic effect prevailed the kinetic factors, which were more pronounced at temperatures below 85˚C
4. Conclusion
A hybrid Mesoporous silica foam impregnated with PEI-10kwas used as a model sorbent to study spectroscopically and gravimetrically CO2 capture. The XPS N 1s peak of the PEI after CO2 treatment was broad with high and low binding energy components that were assigned to N+ of the carbamate. On the other hand, the lower binding energy N 1s component was attributed to CO2 strongly adsorbed to PEI. When the pressure was increased to about 25 bars, the sorption capacity was doubled, probably due to chemical attached and strongly adsorbed (chemisorbed) CO2 to PEI.
Acknowledgements
This paper was made possible by an NPRPGrant#5-1437-1-243 from the Qatar National Research Fund (a member of Qatar Foundation). The statements made herein are solely the responsibility of the authors.
NOTES
*Corresponding author.