Microencapsulation of Ascorbic Acid with Tripalmitin by Using Dry Coating Method ()
1. Introduction
Microcapsules with the many functions have been prepared and applied to the various fields such as drugs, textiles, information recording materials, paintings, cosmetics, food materials, adhesives, medicine, agriculture and so on [1] [2] . The purposes of microencapsulation are to protect the core material from environment, to release the core material according to occasion demands, to handle the gaseous and the liquid core materials as the solid particles and to modify the surface of core material [2] . Moreover, we can give the microcapsules the various functions by how to select the physicochemical properties of the core and the shell materials and how to combine these materials. For examples, there are the combinations of the hydrophilic core materials with the hydrophobic and/or the hydrophilic shell materials and the combinations of the inorganic core materials with the organic and/or the inorganic shell materials. Also, we can select the shell materials with the various physicochemical properties such as magnetism, electric conductivity, thermal conductivity, chemical resistance, biodegradability and so on [3] -[10] .
Ascorbic acid is well known as a redox initiator for polymerization of styrene and methyl methacrylate. If the redox initiator could be microencapsulated and added into these monomers beforehand, polymerization could be induced by breaking the microcapsules due to appropriate stimuli. In dental treatment using polymerization stated above, it has been strongly desired to microencapsulate powdery ascorbic acid with the hydrophobic material. Namely, if ascorbic acid as a redox initiator for polymerization could be microencapsulated with the hydrophobic and chemical resistance materials, the microcapsules could be added into monomer such as styrene and methyl methacrylate beforehand and polymerization should be induced by breaking microcapsules due to heating. As a result, it may be expected that dental treatment has to be extremely improved.
In general, it is well known that the preparation method by using the water phase is not suitable to microencapsulation of water soluble core material [11] -[15] . For example, as the water soluble core materials dissolve in the water phase during the microencapsulation process, the content of core materials may be decreased. Accordingly, it is desirable that the preparation method without the water phase can be applied for microencapsulation of the water soluble materials and the hydrophilic materials. So, it is necessary to develop the method without water for preparing the microcapsules containing the water soluble materials or the hydrophilic materials.
In this study, it is tried to microencapsulate powdery ascorbic acid with tripalmitin as the hydrophobic thermal responsible shell material by using the dry coating method, in which the core and the shell materials are mixed in the mill pot together with pulverizing solvent and the surface of core material is coated with the shell material.
The purposes of this study are to establish the preparation method of microcapsules containing the water soluble ascorbic acid, to investigate how the operational conditions such as the added amount of shell material, the concentration of ethyl alcohol of pulverizing solvent and the coating time affect the characteristics of microcapsules and to apply the microcapsules to polymerization of methyl methacrylate.
2. Experimental
2.1. Materials
Water soluble core material was L-(+)-ascorbic acid (LAA) with the mean diameter of 20 μm (Wako Pure Chemical Industries, Ltd.). Tripalmitin (TP) with the melting point of 80˚C was used as the hydrophobic shell material, which was purchased from Kanto Chemical Co., Inc. Ethyl alcohol (EtOH) (Kanto Chemical Co., Inc.) was used as the pulverizing solvent for the shell material. Methyl methacrylate monomer (Kanto Chemical Co., Inc.) was used to investigate whether the microcapsules could induce polymerization or not.
2.2. Preparation of Microcapsules
Figure 1 and Figure 2 show the schematic diagram of experimental apparatus and the flow chart for preparing the microcapsules, respectively. The unmicroencapsulated active materials are easily deactivated by oxygen or humidity in environment. If the active materials could be microencapsulated by the materials with the barrier ability to oxygen or humidity, the activity of microencapsulated materials should be kept for long time. In microencapsulation of the core materials deactivated easily by oxygen or humidity, it is necessary to develop the microencapsulation method without air and humidity. Taking these things into consideration, we have adopted the dry coating method which is performed under nitrogen atmosphere using only the pulverizing solvent.
First, in order to make sure of the shell formation, LAA and TP were premixed for ten min at the revolution speed of 60 rpm in the semi mill pot as shown in Figure 1(a) by adding the given volume of EtOH. In the semi mill pot, ten nylon balls with the diameter of 7 mm were added. Then, the three semi mill pots containing the mixture of LAA and TP were set in the mill pot to increase the production amount of microcapsules as shown in Figure 1(b) and mixed for the given time at the revolution speed of 60 rpm. Microencapsulation may progress in turn partial solution of TP with EtOH, adhesion of TP and film formation on the surface of LAA particles. In the preparation method of microcapsules presented here, the concentration of EtOH (CE: wt% to TP), the added amount of TP to change the feed ratio (R) of TP to LAA, the size of LAA and the coating time were mainly changed. Table 1 shows the experimental conditions adopted in this study.
![]() (a) (b)
(a) (b)
Figure 1. Schematic diagram of experimental apparatus. (a) Semi mill pot; (b) Mill pot.
![]()
Figure 2. Flow chart for preparing microcapsules.
2.3. Characterization of Microcapsules
Microcapsules prepared as stated above were characterized about the following things:
2.3.1. Observation of Microcapsules and LAA
The surface and morphology of a microcapsule and the LAA particles were observed by scanning electron microscope (SEM) (VE-9800, Keyence Corp., Osaka, Japan).
2.3.2. Mean Diameters of Microcapsules and LAA
The diameters of microcapsules (dp) and LAA particles (DA) were measured by the sieve classification method.
2.3.3. Water Proof Degree
First, LAA of a given weight was dissolved into distilled water of 40 cm3 and then, the concentration of NaOH to neutralize the LAA aqueous solution was measured by titrating with 0.02 mol/L NaOH aqueous solution. From these results, the correlating curve between the concentration of LAA and that of NaOH was obtained beforehand. Then, the microcapsules of 0.5 g were added into distilled water of 40 cm3 to be dispersed for 30min by mixing gently with the magnetic stirrer, under which conditions any microcapsules were not broken mechanically. After this operation, the given volume (10 cm3) of aqueous solution, in which the microcapsules were dispersed, was sampled out and then, the concentration of NaOH to neutralize this solution was measured. By comparing the measured concentration of NaOH with the correlating curve, the concentration of LAA leaking from the microcapsules was estimated. Moreover, the remaining microcapsules were redispersed in distilled water of 40 cm3 and broken by heating up to 80˚C to completely dissolve LAA microencapsulated. Thus, the weight of LAA not leaking from the microcapsules was estimated in the same manner as stated above. From these values, the water proof degree (A) was calculated by using the following Equation (1).
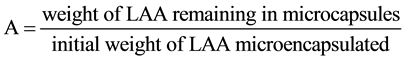 (1)
(1)
2.3.4. Content, Microencapsulation Efficiency, Yield
The content (WC) of core material, the microencapsulation efficiency (λ), the yield (Y) were calculated by using the following equations from the measured values.
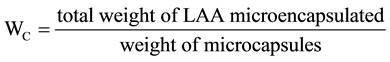 (2)
(2)
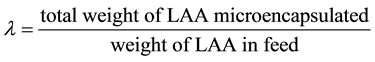 (3)
(3)
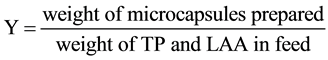 (4)
(4)
2.3.5. Application of Microcapsules to Polymerization of MMA
The microcapsules (0.5 g) were added into the test tube, in which 10 cm3 of MMA monomer dissolving potassium persulfate (KPS) was poured beforehand. It was observed whether polymerization could be induced by heating the test tube at 130˚C and breaking the microcapsules or not. For comparison, the same experiments as stated just above were conducted by adding only LAA with heating at 50˚C and by adding the microcapsules with heating at 50˚C, respectively. The microcapsules were not broken at 50˚C.
3. Results and Discussion
3.1. Effect of Diameter Ratio of LAA to TP
Figure 3 shows the SEM photographs of LAA particles with the different diameters. The mean diameters (DA) of LAA particles are 250 µm (a), 200 µm (b), 150 µm (c), 21.5 µm (d), respectively. While, the mean diameter (DS) of TP particles was 20 µm. The LAA particles are irregular and wide distribution.
Figure 4 shows the effect of ratio of mean diameters (DA) of LAA particles to those (DS) of TP particles on the characteristics of microcapsules where the feed ratio (R) of LAA to TP was 5/5 and the coating time was 6h. With increasing the values of ratio, namely, the number of TP particles per a LAA particle, the yield (Y), the microencapsulation efficiency (λ) and the water resistance degree (A) considerably increased. Here, on supposing that LAA particles are spherical particles, the surface area of LAA particles with the diameters of 250 µm, 200 µm and 150 µm become equal to in turn 0.09, 0.11, 0.14 times of that of LAA particles with the diameter of 21.5 µm. Accordingly, the larger the amount of TP per a LAA particle, the denser the TP shell.
![]()
Figure 4. Effect of ratio of diameters of LAA to that of TP.
On the other hand, the content (WC) was kept almost constant (WC = 0.4) and close to the calculated content (WC = 0.42) based on the feed ratio of LAA and TP. The mean diameters of microcapsules increased according to the diameters of LAA particles.
3.2. Effect of Coating Time
Figure 5 shows the SEM photographs of microcapsules taken each at elapsing time where the diameter ratio (DA/DS) of LAA particles to TP particles was 10.0 and the feed ratio (R) of LAA to TP was 5/5. It was found that the adhesion amount of TA on the LAA particles increased with the coating time and the denser shell was formed.
Figure 6 shows the dependences of the yield (Y), the water proof degree (A), the content (WC) and the microencapsulation efficiency (λ) on the coating time. The microencapsulation efficiency (λ) rapidly increased
2 h 4 h 6 h 8 h 10 h
![]() (a)
(a)![]() (b)(DA/DS = 10, CE = 0, R = 5/5)
(b)(DA/DS = 10, CE = 0, R = 5/5)
Figure 5. SEM photographs of microcapsules (effect of coating time).
from 40% to ca. 100% after 6 h. The water proof degree (A) increased from 0 at t = 2 h to 80% at t = 10 h. The content (WC) increased from 18 at t = 2 h to ca. 40%. The yield (Y) increased from 59% at t = 2 h to 100% at t = 4 h.
3.3. Effect of Concentration of Pulverizing Solvent
Figure 7 shows the SEM photographs of microcapsules prepared by changing the concentration of pulverizing solvent at t = 10 h where the diameter ratio of LAA particles to TP particles was 10.0 and the feed ratio of LAA to TP was 5/5. With increasing the concentration of pulverizing solvent, the adhesion amount of TA increased and the denser shell was formed.
Figure 8 shows the dependence of the yield (Y), water proof degree (A), the content (WC) and the microencapsulation efficiency (λ) on the concentration of pulverizing solvent. The yield (Y) reached 100% at CE = 1.3 vol%. The content (WC) gradually increased and reached 40% at CE = 1.3 vol%. The water proof degree (A) and the microencapsulation efficiency (λ) gradually increased from 79% at CE = 0 wt% to 90% at CE = 2.0 wt% and
![]() (R = 5/5, t = 10 h, DA/DS = 10.0)
(R = 5/5, t = 10 h, DA/DS = 10.0)
Figure 7. SEM photographs of microcapsules (effect of concentration of EtOH).
![]()
Figure 8. Effect of concentration of EtOH.
from 61% at CE = 0 wt% to 82% at CE = 2.0 wt%, respectively.
3.4. Effect of Feed Ratio
Figure 9 shows the SEM photographs of microcapsules prepared by changing the feed ratio (R) of TP to LAA at t = 6 h, DA/DS = 10 and CE = 1.3 wt%. It was found that the larger the ratio, the denser the shell become.
Figure 10 shows the dependences of the yield (Y), the water proof degree (A), the content (WC), the microencapsulation efficiency (λ) on the feed ratio (R). The yield (Y) become 100% at each feed ratio. The microencapsulation efficiency (λ) over 90% could be obtained at each feed ratio. The content (WC) increased with decrease in the feed ratio because of decrease in the amount of shell material in a microcapsule.
![]() (DA/DS = 10.0, t = 6 h, CE = 1.3 wt%)
(DA/DS = 10.0, t = 6 h, CE = 1.3 wt%)
Figure 9. SEM photographs of microcapsules (effect of feed ratio).
3.5. Application of Microcapsules to Polymerization of Methyl Methacrylate Monomer
Figure 11 shows the photographs of the polymerization system of MMA monomer, where the microcapsules prepared at DA/DS = 10, CE = 1.3 wt%, R = 5/5, t = 10 h were used. The monomer phase was solidified by adding unmicroencapsulated LAA at T = 50˚C and by breaking the microcapsules due to heating at 130˚C, but was not solidified by adding the microcapsules at T = 50˚C because of lower temperature than the melting point of
t Only LAA at 50˚C Breakage of Microcapsules at 130˚C Microcapsules Without breakage at 50˚C
![]() (a) (b) (c)
(a) (b) (c)
Figure 11. Application of microcapsules to polymerization.
TP. From this result, it was confirmed that LAA was microencapsulated well and could induce polymerization of MMA monomer.
4. Conclusions
It was tried to microencapsulate LAA with TP by using the dry coating method. The fundamental results were obtained as follows.
1) LAA was microencapsulated well with TP.
2) The yield, the content, the water proof degree and the microencapsulation efficiency increased with the coating time.
3) The yield, the microencapsulation efficiency, the content and the water proof degree increased with the mean diameter ratio of LAA particles to TP particles, the feed ratio of LAA to TP and the concentration of pulverizing solvent.
4) It was confirmed that the microcapsules were broken due to heating at T = 130˚C and could induce polymerization of methyl methacrylate monomer.
NOTES
*Corresponding author.