Changes in Soil Macronutrients after a Long-Term Application of Olive Mill Wastewater ()
1. Introduction
Mediterranean countries are the major world producers of olive oil with 2,205,300 t produced by EU member states in 2011 [1] . The extraction process of olive oil requires a high amounts of water and, correspondingly, large amounts ( more than 30 million m3) of a waste known as olive oil mill wastewater (OMW) is generated [2] [3] . The wastewater is produced over a short lasting period during the winter time, from November to February [4] , creating a major environmental problem. This volume of OMW produced is strongly influenced by the milling method [5] [6] .
OMW is known as recalcitrant effluent which characterized by an acidic pH (4 - 5.5), a very complex redox system (conductivity: 6000 - 16,000 µS), a high buffer capacity, tension activity and stability [7] . More importantly, OMW presents high values for BOD5 (40 - 95 g∙L−1), COD (50 - 180 g∙L−1), LD50 toxicity for fish 8.7%. In addition, OMW contains large amounts of suspended solids and high concentrations of polyaromatic compounds e.g. simple phenols and flavonoids, or polyphenols from 0.5 to 24 g∙L−1 [8] - [11] while it cannot be directly disposed into domestic wastewater treatment. Furthermore, this effluent has a high potassium level and notable concentrations of sodium, phosphorus, calcium and magnesium [3] .
To reduce OMW environmental impact, different remediation methods have been developed such as evaporation in storage ponds, physico-chemical and biological treatments [6] [11] . These include its application in agriculture soils as fertilizer. The high content of organic matter and plants nutrients makes OMW a low-cost fertilizer, as well as a convenient source of irrigation water in Mediterranean countries suffering of water scarcity [12] . Chartzoulakis et al. [13] showed that after 3 years of raw OMW application, there were no significant differences in pH, electrical conductivity (EC), P, Na and organic rates between the control and OMW treated soils. Further, advantage of OMW application is the increase of soil aggregate stability [14] . Nevertheless, this practice creates until now subject to great controversy between its fertilization properties and those effects to its acidity, salinity and phenolic compounds. Therefore, extensive investigations have focused on the change of salinity, pH and hydraulic conductivity, and on the accumulation of phytotoxic polyphenolic compounds inhibiting soil microbial activity [15] - [19] . Mekki et al. [16] noticed the presence of phenolic compounds at a depth of 1.2 m of soil treated with OMW after four months. Moreover, Mekki et al. [16] detected a phytotoxic residual phenolic fraction which was extracted from the top soil layer one year after OMW application. In fact, Mechri et al. [20] showed the inhibition of the arbuscular mycorrhizal fungal root colonization by phenolic compound fraction on reducing the nutrient uptake of the olive trees. Di Bene et al. [21] showed that a long-term repeated OMW spreading has no residual effects or negative trends in soil chemical and biochemical changes. Furthermore, Magdich et al. [22] displayed an improvement of olive yield after the application of OMW on soil for successive 6 years.
The present study involves the effect of annual application of three OMW doses (50, 100 and 200 m3∙ha−1); at different successive layers on sandy soil. We investigate somephysico-chemical soil properties such as sodium adsorption ratio (SAR), exchangeable sodium percentage (ESP) and phenolic compounds dynamics. This study was carried over nine years period in olive field located at experimental station “Chaâl” (Sfax, Tunisia).
2. Materiel and Methods
2.1. Soil and OMW Samples
The soil samples were taken from an agriculture area. The field “Chaâl” is located in experimental station, at 60 Km South-West in Sfax region (Tunisia, latitude North 34˚3', longitude East 10˚20'). The climate of the region is typical Mediterranean with a mean rainfall of 210 mm∙year−1, the average temperature around 27.8˚C in summer and 11.1˚C in winter. The olive-trees field for experimental purposes was divided into four plots (T0, T50, T100 and T200). The latter three were regularly spread with the same annual dose of raw OMW on each January for nine successive years. The experimental plots T50, T100 and T200 have been respectively irrigated with 50, 100, and 200 m3∙ha−1 of untreated OMW [16] . The plot non-irrigated with OMW (T0) served as control.
Ten months after every OMW amendment, soil samples were collected at different layers 0 - 20, 20 - 40, 40 - 60 and 60 - 80 cm for each plot. The field-moist soil samples were sieved (<2 mm), delivered and then stored at 4˚C prior to analysis.
OMW was taken from evaporation ponds at the extraction factory plant in Chaâl, stored at −20˚C and then characterised accordingly before application.
2.2. Physico-Chemical Analyses of OMW and Soil
The OMW characteristics depend on the olive variety, climate and the oil extraction method [23] . The principal characteristics of raw OMW applied on soil were determined by standard method in triplicate. The chemical oxygen demand and biochemical oxygen demand were determined according to AFNOR T 90-101. Total phosphorus P was measured colorimetrically [24] and total nitrogen was determined by the Kjeldahl method. The organic matter was measured after the incineration of samples at 550˚C for 4 h (AFNOR NFV 18-101) and K+, Na+, Ca2+ and Mg2+ by atomic absorption spectrophotometry (HITACHI Model Z-6100). Total phenolic compounds value was determined by using the Folin-Ciocalteau method [25] . The result was expressed in ppm by reference to a standard curve using gallic acid solution. The physico-chemical characteristics of OMW are summarized in Table 1.
Soil analysis for pH, EC, Na, K, Ca, Mg and organic matter (SOM) was performed in triplicate at four differents depths: 0 - 20; 20 - 40; 40 - 60 and 60 - 80 cm. Soil texture analysis was determined using the standard pipette method [26] . The pH and EC were measured on mixture of soil water (1:2.5 and 1:5, respectively). Walkley-Black method was used for the soil organic matter analysis [27] ; P by Olsen and Sommers methods (1982). Total nitrogen was determined as mentioned above. The Exchangeable bases (Na, K, Caand Mg) were determined by atomic absorption spectrophotometry (HITACHI Model Z-6100).
The exchangeable Na+ percentage (ESP) was calculated from exchangeable cations (1) and Sodium Adsorption Ratio (SAR) was determined from Na+, Ca2+ plus Mg2+ (2) in the soil solution [28] .
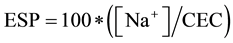 (1);
(1);
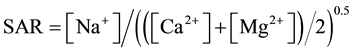 (2).
(2).
The calcium/magnesium (Ca/Mg) ratio was found by dividing the quantity of calcium (cmol+・Kg−1) by the quantity of magnesium (cmol+・Kg−1).
![]()
Table 1. Characterization of OMW used in spreading.
Polyphenolic compounds from the soil samples were extracted with ethyl-acetate. The soil polyphenols were monitored as follows: the samples were extracted with ethyl-acetate using a ratio of 5:1 (v/w). The collected organic fraction was dried at 40˚C in a rotary evaporator. The dry residue was then re-dissolved in methanol and the level of total polyphenol was determined by Folin-Ciocalteau colorimetric method using gallic acid as a standard [25] .
2.3. Field Experimental Soil Characterization
On the toplayer (0 - 20 cm), the soils textures are a sandy (sand 94.3%, clay 4.7%, silt 1.3%) with CaCO3 content about 4.3% accordingly a moderately alkaline pH (7.88). This layer presented a low content of organic matter (0.068%) and cation exchange capacity about 6236 ppm as described in Table 2. The layer 20 - 80 cm was characterized by an alkaline pH (8.22), EC value about 437 µS∙cm−1 and a high content of calcium carbonate (8.3%).
2.4. Statistical Analysis
A minimum of three replicates were used for each analysis and field test. Statistical analysis was performed by using the SPSS 16.0. The treatments means were compared by using the Spearman’s test at 5% significance level.
In order to establish the correlation of the different parameters after OMW amendment, principal component analysis (PCA) was adopted. The PCA is a correlation method transforming the experimental variables data to a set of compound axes, noted principal component (PC). This analysis concerned on ten variables per treatment. For all parameters the Spearman value inferior to 0.05 (p < 0.05) corresponded to correlation coefficient. The correlation matrix between parameters showed a significant interrelation and could be classified into groups.
3. Results and Discussion
3.1. Effect of Raw OMW Application on Soil Physico-Chemical Properties
As a vital component of soil fertility, the soil chemical property reflects its potential ability to provide nutrients for plants [29] . In experimental site, a long-lasting repeated OMW spreading determined the impact of long-term on soil physico-chemical parameters: pH, EC, SOM, Na+, K+, Ca2+, Mg2+ and polyphenols contents in comparison with the control.
3.1.1. pH and EC Progress
A regular application of three doses 50, 100 and 200 m3∙ha−1 of OMW were amended in châal site. The evolution of pH and EC were determined at different successive layers as shown in Table 3. The soil pH indicated no sig- nificant difference with the application of increasing OMW doses. Indeed, the pH variation (7.5 - 8) for treated soil showed no difference compared to the soil taken as reference. The soil pH remains unchangeable despite the pH acidic of OMW (4.63) mainly due to the presence of organic acids. Chartzoulakis et al. [13] noticed no
![]()
Table 2. Characterization of Chaal soil sample.
![]()
Table 3. Effect of a raw OMW on pH and EC soil changes.
significant difference in soil pH after 3 years of successive OMW spreading. This result could be explained by the buffering capacity of the soil which counterbalance the negative effect of OMW [13] . Contrary to the work of Di Bene et al. [21] , the soil pH variation was noticed between control and treated soil after 6 months of amendment. Whereas, Piotrowska et al. [30] also detected a small pH variations after 2 weeks of treated soil.
Table 3 showed the evolution of EC after the spreading of OMW doses, The EC value for the untreated soil registered in the top layer (0 - 20 cm) was 595 µS∙cm−1. For the treated soil, these values were 4, 5 and 9 times higher than the control for each doses applied. Sierra et al. [18] showed the raise of soil EC with increasing OMW rates and the highest OMW dose applied almost duplicate the control salinity. The increase in soil EC was related to the high salts concentration in the OMW which presented an EC value about 14.53 µS∙cm−1. In addition, EC values decreased with depth due to the infiltration by precipitation that induced a transfer of ions to the groundwater [18] . For the dose 200 m3∙ha−1, the high EC value seemed to be irreversible especially for the treated upper soil layer even nine years of treatment.
3.1.2. SOM, P and N Evolution
The analysis of soil organic matter (SOM) in the experimental plots T0, T50, T100 and T200 which have been respectively irrigated with 0, 50, 100, and 200 m3∙ha−1 of OMW was studied as shown in Figure 1(a). The increase of SOM rate was proportional to the increasing of OMW doses application. The SOM on top layer increased from 0.068% in the control to 0.2%, 0.35% and 0.5% with OMW gradual doses application 50, 100 and 200 m3∙ha−1, respectively. The highest level was recorded at plot T100 and T200 on layer 0 - 20 cm, with a slight migration towards the lower layers compared to the control. It was expected that SOM will increase significantly with depth for longer term OMW spreading especially for plots T100 and T200. Furthermore, Mahmoud et al. [15] observed that SOM increased in the top soil after spreading OMW from 5 to 15 years. In addition to being a direct source of essential plant nutrient, SOM was not only supplied of carbon but also for phosphorus and nitrogen and it was not solely affected by concentration but also by composition, according to Haug [31] . During the humification process, organic matter in OMW is transforming easily degradable organic compounds like simple carbohydrates, fats and amino-acids quickly at the beginning of the process, while more resistant organic substrates such as tannins, cellulose, hemi-cellulose and lignin, only partially degraded and slowly transformed [32] . In fact, SOM is transformed to polymerized polyphenolic compounds. These compounds include humic acid-like substances [33] form colloidal complexes with clay and improve the soil fertility and retain more rainfall water. Furthermore, the SOM influenced available nutrient examples by an increased availability of trace element throng complexation by organic ligands or a decreased intoxicity. Diminishing SOM led to a significant decline in the availability of Zn, Cu, Mn and Fe [34] .
The soil phosphorus (P) on toplayer increased slightly from 52.5 ppm to 64.5, 69 and 77 ppm with the application of gradual doses 50, 100 and 200 m3∙ha−1, respectively (Figure 2(b)). Furthermore, total phosphorus was significantly higher referred to control after 6 months of OMW spreading [21] . The spreading of OMW on soil involved the enrichment of the high soil layers by P with a small quantity which migrated towards the lower
![]() (a)
(a)![]() (b)
(b)![]() (c)
(c)
Figure 1. Effect of OMW amendment on SOM, P and N at four soil layers (0 - 20, 20 - 40, 40 - 60 and 60 - 80 cm). Error bars represent ± one standard deviation from the mean, n = 3.
layers even with a higher dose (200 m3∙ha−1). This result could be explained by Carreira et al. [35] that indicated that pedogenic CaCO3 is the primary geochemical agent in arid ecosystems capable of reducing leaching losses of P, through secondary precipitation of Ca-P minerals and/or strong sorption reaction of P with CaCO3, which has broadened ecosystem implications for the retention of P, within both the soil profile and the landscape.
The evolution of nitrogen (N) on soil after long-term repeated OMW spreading doses showed an increase in the top layer (0 - 40 cm) with increasing of gradual doses (Figure 1(c)). N content in the top layers at T50, T100 and T200 plots were 210, 273 and 343 ppm, respectively. These rates were relatively higher than the values recorded in the control soil (161 ppm). After spreading 0, 80 and 160 m3∙ha−1 of OMW on agriculture plots holding three year old alfalfa crops, Gamba et al. [36] showed that the nitrification activity and the nitrate and nitrite contents were lower in the plots that were treated during the vegetative cycle of the harvest and were negatively correlated with the existence of polyphenols. The result approved that adequate OMW dose applied on soil is an eco-compatible practice and has not a side effect on soil biology.
3.1.3. Mineral Elements Progress
Altough potassium (K+) is not considered as polluant, it is present in OMW with a high concentration about 4300 ppm. Figure 2(a) displayed the evolution of K+ after OMW spreading on the soil. K+ level increased on layer (0 - 20 cm) for plot T50, T100 and T200, referred to control. Whereas, the highest level of K+ (850 ppm) was registered on top layer at plot T200. This level was almost 8 times higher than the control. Whereas, K+ level recorded no difference between treated plots and the control from layer 20 - 40 to 60 - 80 cm. Di Bene et al. [21] mentioned that K+ levels were found to be four to 10 folds higher than the control. Generally, K+ is affected by the soil equilibrium conditions (drying and wetting) and subsequent loss by leaching [37] . Potassium is an essential element for development, plant growth and many plant functions [38] [39] . Potassium availability in soil has a major role in estimating fertilization requirements.
Hence, the sodium (Na+) showed the same evolution recorded for K+ (Figure 2(b)). Na+ level in the soil amended with 50 and 100 m3∙ha−1 registered no difference from layer 0 - 20 to 60 - 80 cm. Notably, the raise of Na+ levels in deeper layers of T200 is related to leachining by infiltration. In addition, the Na+ excess on soil solution was neutralised by CaCO3 or CaSO4, both naturally present in the soil [40] . The neutralisation caused a decrease of Ca2+, and since the soil particles tend to repel each other causing clay dispersy and the collapse of the soil structure.
Figure 3 showed Ca2+ level in the top layers of the control plot was higher than the levels recorded at plot T50, T100 and T200. According to OMW organic matter content that affected the pH soil with the realization of ions H+ due to nitrification (enhanced by the increase in soil microbial populations) inducing Ca2+ loss through leaching and Ca2+ set free are adsorbed onto the cation exchange sites and precipitated as carbonate [41] . Figure 3 also showed that Mg2+ level was highe rat the plot T0 for each layers compared to T50, T100. Moreover, the
![]() (a)
(a)![]() (b)
(b)
Figure 2. Soil Na+ and K+ evolution as a function of amended OMW. Error bars represent ± one standard deviation from the mean, n = 3.
![]()
Figure 3. Effect of OMW amendment on Ca2+, Mg2+ and Ca/Mg ratio at four soil layers (0 - 20, 20 - 40, 40 - 60 and 60 - 80 cm).
high rate was recorded in the upper layers for plot T200. In fact, Mg was retained and adsorbed by soil but considerably less strongly than calcium [37] . The soil irrigated with OMW for many years may cause accumula- tive effect of sodium and displace the calcium and magnesium. Furthermore, a significant factor affecting plant mineral nutrition is the Ca and Mg ratio (Ca/Mg) in the soil. A slight variation was found for Ca/Mg ratio (~8.2) on different layers at control plot (Figure 3). This ratio for plot T100 was about 10.2 and 10.8 on the layer 40 - 60 and 60 - 80 cm, respectively. This increase compared to the control was related to the soil Mg level decrease. On the other hand, the Ca/Mg ratio found at plot T200 decreased on the upper layer referred to control due to the decrease of Ca. Pal et al. [42] attested that Ca/Mg was an important indicator of soil structure stability.
The exchangeable Na+ percentage (ESP) and the sodium adsorption ratio (SAR) were presented in Figure 4. As the resultant of long-term repeated OMW application, the exchangeable Na+ percentage (ESP) value was under 2.1% in each layer and for every dose applied (Figure 4). As expected the highest ESP values was recorded at the upper layer (0 - 20 cm) of soil treated with 200 m3∙ha−1. These results are below the limit of ESP which prevents soil from clay dispersy and can lead to serious physical problems [43] . The effect of OMW applied on the soil lead to an accumulation of Na+ in the soil for that SAR was also determinate (Figure 4). SAR values presented the same progress of ESP values. Eventually, SAR values were far below the limit (>15), established to define saline sodicsoils [44] . Moreover, sodium adsorption should be very low due to the sandy material which is mainly composed of gypsum and had a low cation exchange capacity [45] .
3.2. Phenolic Compounds Changing
Polyphenols are the main limiting factor for spreading OMWs because of their phytotoxic and antibacterial ac- tion. Polyphenols content in OMW used in this study was at level 4200 ppm. The phenolic compound concentra- tion in a sandy soil amended with 50, 100 and 200 m3∙ha−1∙year−1 was shown in Table 4. Phenolic compounds concentration on soil control varied from 2835 to 3010 ppm with depth of 0 - 40 cm and 40 - 80 cm, respectively. Phenolic compounds level was very high in the upper layer for each dose amended, and remained high compared
![]()
Table 4. Amount of soil Polyphenols (ppm) after OMW spreading of different doses: 0, 50, 100 and 200 m3 ha-1 in the two soil layers (0 - 40 and 40 - 80 cm). Means with letters a b c and d indicate a significant difference at p ≤ 0.05.
![]()
Figure 4. Effect of long-term OMW application on SAR and ESP progress.
to the control. The application of raw OMW increased the phenolic compounds in the upper soil layers to values above the levels registered in the deeper layers. This result indicated mobility of phenolic compounds in the sandy soil texture. Besides, the concentration of phenolic compounds decreased substantially with depth and the value was not proportional to the gradual increase of the OMW doses. Sierra et al. [18] found that polyphenols are detected at concentration of 200 ppm, even at the depth of 125 cm. The decrease of phenolic compounds with depth could have been a result of the decomposition or incorporation into the humic fraction of the organic matter present in the soil [5] [17] . Moreover, soil biotic and abiotic response exerted capabilities on degradation of OMW phenolic compounds [46] .
3.3. PCA Analysis and Relationship between Different Parameters
In order to test the relationships between macro-nutrient rates on the soil layers and the gradually applied doses of OMW at 50, 100 and 200 m3∙ha−1∙year−1, a Spearman correlation coefficient was used to quantify the strength of the relationship (Table 5). The pH was a negative significant correlated with P (r = −0.503*), K+ (r = −0.806**), Ca2+ (r = −0.638*) and Mg2+ (r = −0.565*), whereas the SOM was a significant positive correlated to P (r = 0.811**), Na+ (r = 0.872**), K+ (r = 0.768**) and SAR (r = 0.872**). K+ was significantly and positively correlated with Mg2+ (r = 0.542*) and SAR (r = 0.701**) while a significantly negative relationship was calculated for Mg2+ level and Ca/Mg ratio. CE was significantly (p < 0.01) positive correlated with SOM, P and Na. A significantly (p < 0.01) positive correlation was calculated with Na+ and K+ levels.
The different parameters dynamics (pH, CE, SOM, P, Na, K, Mg, Ca, Ca/Mg, and SAR) were statiscally investigated using a principal component analysis (PCA) at the nine repeated OMW application. PCA is a multivariate analysis technique and it is usually applied in environmental and agricultural studies.
This analysis reveals relationships between available nutrients in the soil treated and untreated plots. Based on the components loading after the varimax rotation (Figure 5), the result of PCA showed a component 1 (PC 1)
![]()
Table 5. Spearman Correlation matrix for analytical parameters after nine years OMW application.
*p < 0.05, **p < 0.01.
![]()
Figure 5. Plot of the first two principal components for chemical properties.
and component 2 (PC 2) 69.72% and 14.67%, respectively. The components were considered significant and errors being included the variation and various errors in soil sampling and analysis. This analysis displays the presence of three groups.
The first group, including SOM, P, Na, K, Mg, K, SAR and ESP, showed a positive correlation, justified the mineralization of organic matter to available nutrient for plant growth by the microbial activity and confirm the improvement of soil fertility. The second group including pH and Ca/Mg ratio correlated negatively with group 1 related to the mineralisation of carbon and the subsequent production of OH ions by ligand exchange such as K+, Ca2+ and Mg2+ or to the Na+, brought by this waste which generated NaCO3 of more alkaline hydrolysis than the CaCO3. The third group including Ca2+ showed a positive correlation with group 2 related to the relationship between soil pH, Ca/Mg.
According to PCA analysis, EC, Mg, K, P, SOM, Na and SAR were positively correlated in all soil layers while a negative correlation relationship was found between pH and Ca/Mg in the studied layers.
4. Conclusion
The yearly application of three OMW doses (50, 100 and 200 m3∙ha−1) for 9 successive years improved the fertility of Tunisian sandy soil, offering the opportunity to recycle the various compounds. This paper focused on soil macronutrients, phenolic compounds level, SAR and ESP. These results may confirm that OMW applied on soil show a positive effect due to the high amounts of organic matter and macronutrients present. Therefore, OMW could be used as a low-cost soil amendment and an effective fertilizer. However, for the applied dose 200 m3∙ha−1, it should take into account the cumulative effect of soil salinisation, which would transform the soil into an unproductive one.
Acknowledgements
This research was financially supported by Ministry of Higher Education, Scientific Research and Information and Communication Technologies.
NOTES
*Corresponding author.