Study of Thermolysis of Hydrogenated Carbon Molecules as Products of Fullerenization of Benzene, Xylene, Ethanol and Pyridine ()
1. Introduction
Fullerene and its derivatives (fulleranes, endohedral fullerenes, exohedral adducts and substitutional fullerenes (or heterofullerenes)) are increasingly used as sorbents, catalysts, superconductors, lubricants, polymer composites, antioxidants, nanosensors, anticancer and HIV preparations. Fulleranes (or fullerene hydrides) as the first derivatives of fullerene (obtained through 5 years after the discovery of fullerene) have attracted special attention of experts in the field of hydrogen energy as a supposedly promising and efficient accumulator of hydrogen. It was supposed that the electron deficient fullerene molecules can easily be hydrogenated to form a completely saturated molecule, for example, fullerane С60Н60. However, the first experiments of Haufler [1] (the hydrogenation on the method of Birch-Huckel of the fullerene in tert-butyl alcohol at a temperature of −78˚C in liquid ammonia in the presence of lithium metal) showed that C60 molecule is surprising not only on the structure. Hydrogenation of C60 is carried out either on 30% or on 60% (hydrogen attached 9 or 18 from 30 of the double C=C bonds) to form fulleranes С60Н18 and С60Н36, respectively.
Subsequently numerous attempts to obtain fullerene hydrides with an increased content of hydrogen were undertaken. However, a more high degree of hydrogenation (~73%) of the fullerene is only achieved on the methods, in which an ultrahigh pressure and catalysts were used:
・ two-stage method of the preparation of fulleranes when synthesized on the method of Birch-Huckel fullerane C60H36 in the second stage in addition is hydrogenated on the method of Benkeser (by means of the reduction of it in the presence of lithium in ethylenediamine and tert-butyl alcohol) until to the composition C60H44 (with an admixture of minority fraction of C60H46-C60H48) [2] ;
・ the catalytic hydrogenation of the fullerene molecules in toluene solution by using of the catalyst (5% Ru/C) and hydrogen pressure of 12 MPa up to compositions C60H48-C60H50 [3] ;
・ a direct hydrogenation of fullerite by H2 at superhigh (2 GPa) pressure and increased (>450˚C) temperature during 30 - 90 minutes up to composition C60H44-C60H52 [4] -[6] .
The maximum hydrogen content (corresponding ostensibly to the composition C60H60 [7] ) in the hydrogenated fullerite was achieved only under conditions of long heat treatment (over 10 hours) at temperatures above 450˚C and high pressures (1 - 12 MPa). However, it should be noted that a fullerene structure at the hydrogenation degree of C=C bonds about 80% almost completely disappears: in such hydrogenated fullerene cage can be kept less than 5- (from 30) double bond (or one benzenoid cycle). In conditions of the hydrogenation at so high pressures (~9.8 MPa) and temperatures (>600˚C), synthesized molecules of fullerane almost completely can collapse with forming of smaller fragments C59Hx, C58Hx, C57Hx… as radicals, which, of course, in thermostable predominantly saturated hydrocarbons, are easily hydrogenated. Saturation, destroyed in any of the fragments, for example C57Hx of the C-C bonds by hydrogen, creates the illusion of obtaining of fullerane of equiatomic composition, C60H60. Therefore the process of dehydrogenation synthesized under high pressure of hydrogenation products of fullerites is realized at sufficiently high (above 400˚C) temperatures and is accompanied not by reconstruction of closed fullerene skeleton but its destruction with the formation of the hydrocarbon fragments of different compositions.
Thus, to date all developed methods of synthesis of fulleranes (Figure 1) [1] -[11] are based on a single process―the process of hydrogenation pre-synthesizing fullerene, fullerite (or fulleride palladium). Hydrogen sources [1] -[11] such as polyamines, diimide, methanol, metal hydrides, hydrogen, dihydroanthracene and other hydrocarbons, system Zn-conc. HCl are varied only. Therefore the study of new approaches to develop more efficient method of synthesis, not only fulleranes [12] -[14] , but also quasi-fulleranes [15] -[20] , is extremely urgent.
In this article we present the experimental results demonstrating the possibility of obtaining in gram quantities of the samples of hydrogenated fullerenes (С60 and С70) and different compositions of quasi-fullerenes. Synthesis of hydrogenated carbon molecules was carried by using of other essence methods (distinct from all known methods), since in this method preliminary technological stage of the synthesis of carbon molecules is completely excluded. Fulleranes and quasi-fulleranes, as well as some carbon molecules directly from molecules of aromatic hydrocarbons (benzene, xylene), pyridine and ethanol were synthesized.
Fullerenization [12] [14] [15] , as the process of direct transformation of organic molecules into carbon molecules and their hydrides, is realized at a special method of heat treatment of vapor of precursors. It should be noted that earlier in the products of a new method (аuthor’s method) of pyrolysis (NMP) of pyridine [13] [15] [19] , benzene [12] [13] [17] [18] [20] , as well as o-xylene, toluene and ethanol [14] have been found to have various types of carbon molecules: fullerene С60, quasi-fullerenes С48, С40, С42 and their hydrides as well as small
![]()
Figure 1. Schematic comparison of an author’s method to known [1] -[11] methods of synthesis of hydrogenated carbon molecules (tol―toluene, m―minute, h―hour).
carbon molecules С3-С15. Besides, by means of a mass spectrometric analysis, it was convincingly shown that after processing by the laser of pyrolysis products of pyridine [13] [15] [19] , benzene [16] , toluene, o-xylene and ethanol [14] , the hydrogen already has appreciably allocated. Herein, basic attention will be given on the comparative study by mass spectrometric analysis of gaseous substances (and, first of all, hydrogen) formed at thermal decomposition (in temperature range 30˚C - 750˚C) of hydrogenated carbon molecules, which in the process of fullerenization of organic molecules are synthesized.
2. Experimental Section
In [12] -[20] on the basis of the experimental results the main feature of the NMP was demonstrated: the composition of the obtained products not only from the reaction conditions (temperature, reagent concentration and time of it stay in the most high-temperature zone of the reactor), but and the place of localization of these products in the reactionary space depends. At NMP a part of the condensed substances and resultant soot in a gas reactionary flow from the high temperature reaction zone (regime 1˚C - 1050˚C, regime 2˚C - 950˚C) are taken out and in a low temperature zone (100˚C - 300˚C) of reactionary space are located. The сomposition and an amount of these products from temperature in a high-temperature zone (regimes 1 and 2) and time of stay in it reagents depends. The tests at temperatures (regimes 1 and 2) more favorable for polymerization processes (not dehydropolimerization) with the formation of hydrogenated carbon molecules were carried out. Synthesized at regimes 1 and 2 products of pyrolysis by the first letters of the names of initial reagents are designated. Thus, samples have defined as: pyrolysis products of benzene B1 and B2, xylene X1 and X2, ethanol E1 and E2, pyridine P1 and P2, obtained at regimes 1 and 2 respectively. Here the results of the study of products obtained at NMP of organic compounds vapors and localized only in the low-temperature zone are presented. In this zone along with a deposited carbon also are condensed the carbon molecules of different sizes and their hydrides [12] -[20] . Our preliminary results showed that an allocation of hydrogen from the condensed products of NMP of pyridine [13] , benzene [13] , o-xylene, toluene and ethanol [14] has been observed at temperatures below 100˚C.
The products of several (8 - 10) tests obtained at similar reaction conditions and localized in the low-tempera- ture zone mixed up and averaged. Then the condensed molecules from these samples by benzene and xylene carefully are extracted. The obtained extracts were concentrated and then most part of the dissolved carbon molecules and them hydrides by ethanol were deposited. The obtained powdery precipitations were investigated by chemical and X-ray (DRON-UM diffractometer with CuKα-radiation and nickel filter) analysis, NMR (AVANCE 400 spectrometer (Bruker, Germany)), and IR spectroscopy (Nexus Nicolet FTIR spectrometer (Thermo Scientific)), as well as by temperature-programmed desorption mass spectrometry (TPD MS) of volatile products. Mass spectrometric analysis in the field of up to 200 m/z was carried out on monopole mass spectrometer МХ-7304А (Sumy, Ukraine) with impact electron ionization (EI) [21] . The samples at the bottom of molibdenium-quarts ampoule was evacuated at room temperature up to 5 × 10−5 Pa. The linear heating of a sample up to 750˚C was carried out with speed 0.15 K∙s−1 [21] . The volatile thermolysis products are passed through a high-vacuum valve ( 5.4 mm in diameter) into the ionization chamber of the mass-spectrometer.
Some part of powdery deposits again in toluene was dissolved. Prepared toluene solution on a metal substrate is placed and after evaporation of the solvent by a method of matrix-assisted desorption/ionization (MALDI) mass spectrometry with using spectrometer Bruker Daltonics flexAnalysis (irradiating by nitrogen laser with wavelength of 337 nm) was studied. For the majority of the detected in the mass spectra of molecules fine structure of their peaks was analyzed, as described in detail earlier [13] -[20] , that allowed more correctly to present composition of detected molecules.
3. Results and Discussion
Products of low temperature zone in the form of soot B02, X02, E02, P02, and B01, X01, E01, P01, obtained by the pyrolysis of organic molecules at temperature regimes 1 and 2, respectively, were treated with several portions of an aromatic hydrocarbon to extract soluble in these solvents substances. Color of the extracts of the products obtained by regimes 1 and 2, approximately is the same and is changed from dark red (first treatment) to light yellow (the last treatment). Dark red B2, X2, E2, P2 and light yellow B1, X1, E1, P1 the deposits from the concentrated extracts by means of addition of ethanol were deposited. XRD patterns of these deposits practically are similar and testify about high them amorphousness.
In accordance with the chemical analysis all samples alongside with carbon and hydrogen contain oxygen up to 3.9 mass%. Samples P1 and P2 also nitrogen 3.2 and 2.9 mass%, respectively contain. Hydrogen content in the light yellow samples E1 (5.7 mass%), P1 (5.3 mass%), X1 (5.1 mass%), B1 (4.9 mass%) is significantly higher than in a dark red samples P2 (2.9 mass%), B2 (2.8 mass%).
3.1. Mass Spectrometric Study of the Composition and Thermal Stability of the Condensed Products of Fullerenization of Organic Compounds
All products of organics pyrolysis according to investigations of them thermodesorbtion contain hydrogen, which allocation from obtained at a regime 1 products is began already at temperature below 50˚C (Figure 2) and is proceeded in all investigated temperature interval. It is important to note, that the process of fulleranes dehydrogenation synthesized at hydrogenation fullerite and fullerenes only at temperatures above 400˚C is observed [22] [23] . Comparison of desorbtion thermograms of hydrogen (Figure 2) and mass-spectra EI of volatile products of thermal decomposition of identical mass (3 mg) samples obtained at regimes 1 and 2, shows, that the contents of hydrogen in these samples essentially are differed.
Programmed linear heating of a sample P1 carried out only up to 550˚C however tendency in increase of the amount of allocated hydrogen in process of increase of temperature (curve 1 in Figure 2(a)) is observed rather distinctly. In products obtained at a regime 1, almost in three times of hydrogen is contained more (curve 1 and 2 in a Figure 2(a)), than in products prepared at a regime 2 (curve 3 and 4 in Figure 2(a)). Is remarkable, that curves of temperature dependence of intensity of allocation of hydrogen, (especially from products P1 and P2 of pyridine pyrolysis), have three distinct maxima (~100˚C, 380˚C and 700˚C). This fact can testify to presence in P1 and P2 of three types of hydrogenated carbon molecules, the energy of bonds C-H in which is appreciable different. The higher intensity of maxima at low temperature on thermograms of samples P1 and P2 allows to assume, that products of pyridine pyrolysis, containing also hydrogenated N-heteroatomic molecules (poliazafullerenes) [15] [19] , are less thermostable. By comparison of desorbtion thermograms of hydrogen from all products obtained at a regime 1 (Figure 2(b)), distinctly it is possible to see, that the most intensive allocation of hydrogen in all a researched interval of temperatures from a product of ethanol pyrolysis E1 is observed (curve 4 on Figure 2(b)).
Methane, propane and butane alongside with hydrogen in a gaseous phase of a thermolysis of all products of pyrolysis are detected. Characteristic from 100% by intensity (according to a database of mass spectra National Institute of Standards and Technology USA (NIST)) peaks for CH4, C2H6 and C3H8 are ions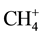 ,
, 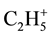 and
and 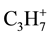 with m/z 16, 29 and 43 accordingly. It is known [23] that the destruction of a fullerene cage at fulleranes dehydrogenation with primary formation of such hydrocarbons as methane and propane at 400˚C is began. Temperature of a beginning of dehydrogenation and partial destruction of fullerene cage appreciably on a method of synthesis of fulleranes depends. So, the dehydrogenation of intercalated by sodium fullerane (Na6C60Hx) obtained at interaction of fullerene C60 with sodium hydride (NaH) is started already at 375˚C [24] . Probably, the sodium atoms in Na6C60Hx decrease the energy of activation of break of bonds С-Н. However the content of hydrogen in intercalated fullerane (Na6C60Hx) is essentially reduced because of presence of sodium [24] . The intensive loss of mass with allocation of hydrogen and hydrocarbons at heating of samples of fullerene hydride С60Н36 obtained by hydrogenation of fullerene both by dihydroanthracene and by Birch method, is started at temperatures 510˚C and 380˚C accordingly [22] . Methane from products of organics pyrolysis begins to be formed at a little bit lower temperature (300˚C) that may be connected with greater particles dispersity of X-ray amorphous of samples. Here it is necessary to note, that the peak with m/z 16 corresponds not only methane, but also is alongside with peaks with m/z 18 and 17 characteristic (with intensity 0.9% concerning peak with m/z 18% - 100%) for mass spectrum of water. However on the thermogram (Figure 3(a)) the temperature interval of allocation of methane (with m/z 16) does not coincide with maxima of peaks with m/z 18 and m/z 17. Besides in mass spectrum of water there is no peak with m/z 15, while for methane it is characteristic with intensity 85.4 % concerning peak with m/z 16 (with 100% intensity). Therefore a presence of peak with m/z 15 to the appropriate intensity, which repeats a configuration of peak with m/z 16, convincingly confirms the allocation of methane from products of pyrolysis (Figure 3(a)). Peaks with m/z 16 on the thermograms of all products obtained at a
with m/z 16, 29 and 43 accordingly. It is known [23] that the destruction of a fullerene cage at fulleranes dehydrogenation with primary formation of such hydrocarbons as methane and propane at 400˚C is began. Temperature of a beginning of dehydrogenation and partial destruction of fullerene cage appreciably on a method of synthesis of fulleranes depends. So, the dehydrogenation of intercalated by sodium fullerane (Na6C60Hx) obtained at interaction of fullerene C60 with sodium hydride (NaH) is started already at 375˚C [24] . Probably, the sodium atoms in Na6C60Hx decrease the energy of activation of break of bonds С-Н. However the content of hydrogen in intercalated fullerane (Na6C60Hx) is essentially reduced because of presence of sodium [24] . The intensive loss of mass with allocation of hydrogen and hydrocarbons at heating of samples of fullerene hydride С60Н36 obtained by hydrogenation of fullerene both by dihydroanthracene and by Birch method, is started at temperatures 510˚C and 380˚C accordingly [22] . Methane from products of organics pyrolysis begins to be formed at a little bit lower temperature (300˚C) that may be connected with greater particles dispersity of X-ray amorphous of samples. Here it is necessary to note, that the peak with m/z 16 corresponds not only methane, but also is alongside with peaks with m/z 18 and 17 characteristic (with intensity 0.9% concerning peak with m/z 18% - 100%) for mass spectrum of water. However on the thermogram (Figure 3(a)) the temperature interval of allocation of methane (with m/z 16) does not coincide with maxima of peaks with m/z 18 and m/z 17. Besides in mass spectrum of water there is no peak with m/z 15, while for methane it is characteristic with intensity 85.4 % concerning peak with m/z 16 (with 100% intensity). Therefore a presence of peak with m/z 15 to the appropriate intensity, which repeats a configuration of peak with m/z 16, convincingly confirms the allocation of methane from products of pyrolysis (Figure 3(a)). Peaks with m/z 16 on the thermograms of all products obtained at a
![]() (a) (b)
(a) (b)
Figure 2. Experimental thermodesorption curves for the 3 amu peaks (appropriate to hydrogen) for samples: 1―P1, 2―B1, 3―P2, 4―B2 (a); 1―P1, 2―B1, 3―X1, 4―E1 (b).
![]() (a) (b)
(a) (b)
Figure 3. Experimental thermodesorption curves for selected 1―m/z 18, 2―m/z 17, 3―m/z 16, 4―m/z 15 peaks for the product X1 (a); Anions mass spectrum MALDI of the product B2 (b).
temperature regime 1 are visible. From these thermograms follows, that the intensive allocation of methane in identical to all samples an interval of temperatures 300˚C - 750˚C to a maximum at ~500˚C is observed. The most intensive methane allocation (the same as also of hydrogen), in a product of ethanol pyrolysis is observed.
At low temperatures (<100˚C) of thermolysis from a surface of all samples the rests of the used aromatic solvents and, probably, products (ions 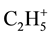 and
and 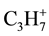 with m/z 29 and 43 accordingly) them fragmentation intensively are desorbed. The allocation of
with m/z 29 and 43 accordingly) them fragmentation intensively are desorbed. The allocation of  and
and 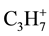 fragments (as against molecules of the solvents) in all the investigated interval of temperatures is observed. However the most intensive allocation of
fragments (as against molecules of the solvents) in all the investigated interval of temperatures is observed. However the most intensive allocation of 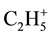 and
and 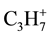 at ~150˚C, 300˚C and 400˚C is occurred, that can be connected with the destruction of hydrogenated carbon molecules. Among the 4-th investigated products most intensive allocation
at ~150˚C, 300˚C and 400˚C is occurred, that can be connected with the destruction of hydrogenated carbon molecules. Among the 4-th investigated products most intensive allocation 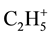 and
and 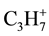 for products of ethanol pyrolysis E1 are characteristic.
for products of ethanol pyrolysis E1 are characteristic.
Thermograms of all investigated products of pyrolysis contain peaks of adsorbed water (m/z 18) and oxygen (m/z 32) as well as carbon oxides (CO (m/z 28) and CO2 (m/z 44)), which, usually, at the thermolysis of fullerenes hydrides and another carbon materials [25] are allocated
A distinctive feature of the products of pyridine pyrolysis (from product the pyrolysis of benzene, xylene and ethanol) is that the peak HCN with m/z 27 in thermogram of product P1 is detected. This fact testifies that in the sample P1 the nitrogen-containing molecules (possibly poliazafullerenes) [15] [19] are contained. Allocation HCN in the process of thermodesorption of sample P1 in all a researched interval of temperatures is observed. Furthermore, the amount of generated HCN with increasing decomposition temperature of the sample P1 continuously is increased, probably, because of the destruction of the nitrogen-containing carbon molecules contained in the product. It should be noted that along with HCN, signal with m/z 27 with the intensity of ~5% also corresponds to one of decomposition products of molecules of benzene and xylene (according to the database of mass spectra of NIST). These solvents in the products of salting out of carbon molecules are always presented (as later will be shown). However, line with m/z 27 on thermogram of sample P1 in the entire investigated temperature range significantly is differed in shape and intensity from the lines with m/z 78 and 106 of molecular ions of benzene and xylene respectively. Notably, that from the pyrolysis products of pyridine, in particular sample P1, most intense desorption of solvents (especially benzene) at both low (~100˚C) and a significantly higher (350˚C) temperatures (humped line of solvent desorption) is observed. Solvents from the samples B1, X1, E1 almost completely at low temperatures are desorbed. At the same time, according to the thermogram of a sample P1 most part of the solvent at higher temperatures (300˚C - 420˚C) is desorbed. Therefore, it is possible to assume that in the process of precipitation (salting out) only pyrolysis products of pyridine (probably nitrogen-containing hydrogenated carbon molecules) with the molecules of solvent sufficiently thermostable crystal solvates form. Consequently, a unique feature of condensed products of fullerenization process of given organic molecules is that the allocation from them hydrogen at temperature below 50˚C is already began (Figure 2) and in all the investigated temperature interval (50˚C - 750˚C) is proceeded. Therefore it is very important to estimate a size, composition and formula of these condensed, as we hope, mainly carbon molecules.
Investigation by MALDI mass spectrometric analysis of the pyrolysis products of xylene, toluene and ethanol, previously presented by us in [14] , showed that their spectra are similar and the reflexes answering to molecules of quasi-fulleranes and fulleranes contain. In the present communication the samples B1 and B2 containing hydrogen from the synthesized pyrolysis products of benzene in the largest and smallest amount accordingly were investigated by mass spectrometry MALDI analysis. Positively and negatively charged clusters are registered. In the mass spectrum of positive ions of product B1 (Figure 4) as and the products X1, E1 [14] , P1 [13] obtained at a high-temperature regime 1 in the range 254 - 574 m/z there is group of intense peaks, having strict periodicity: the values m/z grow serially on 24 or 26 units. In spectra of the high resolution it is possible to see, that the ratios of intensities of peaks 12Cn and 12Cn-113C of ions with m/z 254, 276, 302, 326 (Figure 4, inset), 350 (Figure 4, inset), 376, 400, 424, 450, 476, 500, 524, 550 and 574 correspond to natural isotope distribution of carbon for quasi-fulleranes of following compositions: C20H14, C22H12, C24H14, C26H14, C28H14, C30H16, C32H16, C34H16, C36H18, C38H20, C40H20, C42H20, C44H22 and C46H22. At the same time an intensity of peaks 12Cn-213C2 of these molecules is much higher than the calculated values of carbon isotope distribution that can testify to presence of more hydrogenated molecules, for example, compositions С20H16-C46H24, respectively.
Moreover, on examples of thin spectra presented in the insets (Figure 4, insets) can clearly seen, that each of the main peaks, in reality, contains a series of peaks which can be characteristic for more hydrogenated quasi- fullerene molecules. For example, peaks at m/z 326 and 350 may correspond to a number of quasi-fulleranes of compositions C26H14-C26H24 and C28H14-C28H22 respectively. It is important to note, that at the laser irradiation
![]()
Figure 4. Cations mass spectrum MALDI of the product B1 with the expansion around the m/z 2 - 4; m/z 326, 350 peaks and the calculated isotope mass ratios for С26H14, С28H14 molecules respectively in the insets.
of the sample an extremely intensive dehydrogenation of B1 is observed (an intensity of peaks of negative and positive (Figure 4, inset) ions of hydrogen in several times is higher than the intensity of other peaks in this mass spectrum). Therefore it is possible to assume, that more soft methods of mass spectrometry can detect the clusters more hydrogenated of quasi-fulleranes.
In MALDI mass spectra of products X2, E2 [14] , P2 [13] and B2 [12] with lower hydrogen content the peaks corresponding to the hydrogenated quasi-fullerenes, practically are not detected. However, in the spectrum of negative ions, for example, of product B2 (Figure 3(b)) clearly there are peaks which for hydrides of fullerenes C60 (C60H8, C60H18, C60H32, C60H46, C60H60) and С70 (C70H8, C70H22, C70H36, C70H44) are characteristic. Notably, in the spectrum of positive ions the clusters of these fulleranes are not detected. As was established by us previously [12] -[20] , the clusters of small carbon molecules and their hydrides in the form of anions the most clearly are detected.
3.2. Investigation of IR and NMR Spectra of Condensed Products of Pyrolysis of Organics
The structures of IR spectra of powders P1, B1, X1, E1 and P2, B2, X2, E2, obtained from products of pyrolysis of organics at regimes 1 and 2 accordingly, basically are similar and remind structures of IR spectra of fulleranes of different compositions prepared by hydrogenation fullerenes. So, the values of the basic absorption bands in IR spectra of samples X2, B2 and P2 are rather close to values established for fullerane С60Н36 [1] [8] [10] [11] as well as to calculated value for hypothetical С60Н60 [26] (Table 1). (In the table designations of types of vibrations are used standard: ar―aromatic, st―stretching, δ―bending, ip―in plane, oop―out of plane, (1-9) F1u― active vibration modes of bonds C-H and С-С calculated for fullerane С60H60 [26] .) Partially hydrogenated fullerene molecules (for example C60H36) have a lower symmetry than fullerene C60 (which has 4 modes with symmetry F1u only) and fullerane of an equiatomic composition C60H60, which has 9 active modes [26] (Table 1) three of which are concerned to the vibrations of C-H bonds, and 6―to vibrations of the fullerene cage. So, IR spectra of partially hydrogenated fullerenes have a somewhat larger number of bands. According to the structure of the IR spectra of the samples X2, B2, and P2 it is possible to assume that given pyrolysis products contain mainly of hydrogenated carbon molecules. The absorption bands in the IR spectra of the samples X2, B2 and P2, probably responsible for the distorted spherical cage of carbon molecules have values (1596, 1449, 1154, 752, 456 cm−1), (1600, 1453, 1145, 830, 698, 537 cm−1) and (1594, 1461, 1166, 754, 453 cm−1) respectively.
Some absorption bands of samples X2, B2 and P2 to the calculated values of the absorption bands of the carbon cage of fullerane C60H60 [26] are closest. According to mass spectrometric investigation [12] condensed
![]()
Table 1. Comparison of the main absorption bands in the IR spectra of samples X1, B1, P1 and C60H36 [1] [8] [10] [11] and calculated [26] for C60H60 values of the absorption bands.
products of benzene pyrolysis contain fulleranes with high hydrogen content, such as С60Н46, С60Н60 and С70Н36. Absorption bands at752 cm−1, 747 cm−1 and 754 cm−1, observed in IR spectra of X2, B2 and P2 samples accordingly, are close to values 744 and 730 cm−1, which for samples of fullerane C60H36, obtained by hydrogenation fullerene with the use С2Н5J [27] or Zn + conc. HCl [10] , are characteristic. The absorption bands at 1596 cm-1 (X2), 1600 cm−1 (B2) and 1594 cm−1 (P2) can correspond to bonds C=C in partially hydrogenated fullerane. Amount of bands of absorption in the field of 1350 - 1700 cm− 1 in a IR spectrum samples P1 (received from pyridine) are more a little in comparison with IR spectra of samples B2, X2, (received from aromatic hydrocarbons). The presence of atom (atoms) of nitrogen into a molecule, for example, hydrogenated polyazafullerenes [15] [19] changes not only a quality of bonds but also their amount. To presence of nitrogen-containing molecules in the product P2 testifies also the characteristic for a C≡N absorption band at 2222 cm−1.
Characteristic for bonds C-H stretching at 2920 (2922, 2922) and 2843 (2853, 2853) cm−1 and bending at 1366 (1362, 1377) cm−1 vibrations in the IR spectra of samples X2 (B2, P2) are satisfactory coordinated with IR spectra of fullerene hydrides obtained by different methods [1] [8] [10] [11] [27] (Table 1). However, the values of the absorption bands of C-H bonds are especially close for samples B2, P2 and fullerene C60H36 obtained by the method of Birch-Huckel [1] [10] (Table 1). Low-intensity absorption band at ~3050 cm−1 characteristic for stretching vibrations of aromatic C-H bonds, testifies to presence in the products of solvated aromatic solvents (benzene and xylene). Thus, the structure of the IR spectra of pyrolysis products of organics obtained at temperature regime 2, practically coincide with the structure of the IR spectra of various compositions of fulleranes obtained by hydrogenation of fullerenes.
In 13C NMR spectrum of the product of pyrolysis pyridine P2, dissolved in hexamethyl phosphoramide (HMPA), two groups characteristic for fullerene hydrides [10] [22] [28] signals in the region 25 - 50 ppm and in the region of 125 - 140 ppm as well as the signal of HMPA at δ 37.086 ppm clearly are visible. Signals of carbon atoms of the aromatic ring are much weaker (probably due to the quaternary nature of these atoms) that also is characteristic for partially hydrogenated fullerenes [10] . At more detailed consideration of signals in the C-sp2, the complex multiplet with values δ 124.555, 128.184, 128.902 and 129.693 ppm, as well as weak signals with δ 134, 137 and 139 ppm are visible. Plenty of signals may indicate that the product P2 contains hydrogenated carbon molecules of different composition.
At the signals of C-sp3, in addition to the most intense signal characteristic for fulleranes at δ 26.642 ppm and a for solvent at δ 37.086 ppm, weaker signals at δ 27, 30, 35, and 45 ppm are observed, which can correspond to quasi-fullerenes and hydrogenated small carbon molecules.
At the 1H NMR spectrum of the sample P2 the most intensive signals with a δ 2.484, 2.490, 2.580, 2.604, 3.579 and 4.849 ppm are present, which to fullerene hydrides usually are characteristic [10] [22] [28] . Notably, that the signals in the region δ 2.1 - 2.5 ppm observable also in a spectrum 1Н NMR of a sample P2, can correspond to fulleranes as suppose in [10] , with the greater degree of hydrogenation, than well investigated fullerane С60Н36. Weak signals at δ 7 - 8 ppm may be associated with the presence of aromatic solvents, which, are involved in the formation of crystal solvates. It should also be noted that signals from the sample by the intense signal of the solvent (HMPA) with δ 2.517 ppm are overlapped.
Thus, the presented experimental results convincingly testify, that fullerenization of molecules of organic substances in molecules of quasi-fulleranes (C20-46H14-44) and fulleranes (C60H4-C60H60 and C70H36-C70H8) at the special method of pyrolysis is observed. In process of fullerenization of molecules of xylene, toluene, ethanol, benzene and pyridine the carbon molecules as quasi-fullerenes (C40, C48 и C42), fullerene (C60) and small molecules C3-C11 are formed also [12] -[20] . A feature of the pyrolysis of pyridine is that the nitrogen-containing fullerene-like molecules [15] [19] and nanostructures [29] [30] are formed also. It is remarkable, that earlier the small carbon clusters in carbon plasma were detected only. The presence of small carbon molecules in products of the pyrolysis, undoubtedly, testifies that at author method of pyrolysis in parallel with reactions of polymerization and dehydropolymerization (polycondensation) the reaction of the destruction of molecules of precursors is realized. Small carbon of molecules (C3-C11), probably, can be intermediate ones at formation of fullerene and quasi-fullerenes from molecules investigated precursors. Fulleranes and quasi-fulleranes by reactions of polymerization of precursor molecules and the product of their destruction can be formed.
4. Conclusions
1) A fundamentally new method of synthesis of hydrogenated carbon molecules is developed, wherein the step of pre-fullerene synthesis inherent to all known methods of fulleranes producing is excluded completely.
2) First the reaction conditions for direct transformation (fullerenization) molecules of organic compounds in fullerene hydrides (С60Н8-С60Н60 и С70Н8-С70Н44) and quasi-fullerenes hydrides (CnHn-6-CnHn-2 (n = 20 - 46)), containing up to 5.7 wt% hydrogen are created.
3) First it is shown that the allocation of hydrogen from hydrogenated carbon molecules can start with such low (~50˚C) temperatures.
4) First it is established that quasi-fulleranes and fulleranes that are synthesized by pyridine pyrolysis under their precipitation by molecules of aromatic solvents, form the crystal solvents that are thermostable up to 350˚C.
NOTES
*Corresponding author.