1. Introduction
Heavy metals, such as Cr(VI), Cu(II), Pb(II), Zn(II), Cd(II), etc., are increasingly being discharged into surface water. They are non-degradable and tend to accumulate along the food chain. Many heavy metal ions are known to be toxic or carcinogenic. Lead(II) is one of the most toxic heavy metals and exists in the waste water of many industries like planting, tanneries, oil refining and mining [1] . The permissible levels of lead in drinking and waste water are 0.005 mg∙L−1 and 0.05 mg∙L−1, respectively [2] . Lead can affect almost every organ and system in the body. Lead is a substitute for calcium in bony tissues and accumulates there. Exposure to high lead levels can severely damage the brain and kidneys and ultimately cause death [3] , hence, the removal of Pb(II) from water is important for the protection of public health and the environment. Several methods such as chemical precipitation [4] , electrochemical reduction [5] , ion exchange [6] , reverse osmosis [7] , and membrane separation [8] have been developed to remove Pb(II) from waste water. However, these technologies are either expensive for the treatment and disposal of the secondary toxic metal sludge, require excessive time for successful treatment, have high energy consumption or are ineffective when Pb(II) is present in the wastewater at low concentrations. In contrast, adsorption technique is one of the preferred methods due to its simplicity as well as the availability of a wide range of adsorbents [9] . In principle, any solid material with a micro porous structure can be used as an adsorbent. The most important property of any adsorbent is the surface area and structure. Furthermore, the chemical nature and polarity of the adsorbent surface can influence the attractive forces between the adsorbent and adsorbate [10] . Zeolites are among the most commonly used adsorbents. Zeolites are a large group of natural and synthetic hydrated aluminum silicates. They are characterized by complex three-dimen- sional structures with large cage like cavities that can accommodate various pollutants and even small organic molecules. Ions and molecules in the cages can be removed or exchanged without destroying the aluminosilicate framework. The structure of zeolites allows for their wide usage as adsorption media for a large number of organic and inorganic materials, including trace concentrations of heavy metals.
The process of adsorption using zeolites is affected by a number of factors including the temperature, pH, contact time and concentration of the pollutant. The pH of aqueous solutions and wastewater effluentsin partic- ular, affects a number of reactions that takeplace in these solutions [11] [12] . The effect of pH on heavy metal adsorption from aqueous solutions has been reported [12] - [14] . It was found that the removal of heavy metal ions was pH dependent at the different pH ranges that were tested. Each result presents a characteristic variation of pH with amount adsorbed depending on the adsorbent type, metal ion and/or initial concentration of metal ions; however, limited studies are available of the pH dependence of zeolites. Specifically, studies on the pH dependence of heavy metal adsorption on such zeolites as A4, whose structural stability is also pH dependent. It has been observed that zeolite A4 has an unstable structure with pH changes. Low acidity (pH below 4) may collapse zeolite structure, especially those with low Si/Al ratio, but the destruction will be more at pH below 3 [15] . Ribeiro [16] reported that the exchange of Linde type A4 with multivalent ions presents the most difficult task experimentally because of the limited stability of this zeolite at low pH levels. This study was therefore conducted with the purpose of understanding the pH dependence of heavy metal adsorption specifically lead.
2. Materials and Methods
2.1. Zeolite Samples
The choice of zeolite samples for the experiments was based on the variations in their Cation Exchange Capacities (CEC), the difference in their pore structures as well as their availability. Commercially available zeolite A4, faujasite X, faujasite Y and mordenite were used for the experiments. Zeolites A4 and faujasite X were supplied by Wako Pure Chemicals Industries Japan, faujasite Y was supplied by Tosoh Company, Japan and natural mor- denite from Fukushima Prefecture in Japan. The above samples were used with Na+ saturation in order to have Na+ as the uniform exchangeable cation.
2.2. Sample Characterization
The samples were characterized by powder X-ray diffraction (XRD) using a Rigaku Ultima IV X-ray Powder X- ray diffractometer with Cu-Kα radiation generated at 40 kV and 40 mA, between 3˚ - 60˚ of 2θ angles with a sampling width of 0.02˚ and a scanning rate of 2˚∙min−1. Cation exchange capacity (CEC) was measured by initial saturation of samples with 1 M KCl solution. The K+ saturated samples were washed with 1 M NH4Cl solution in order to remove the exchangeable K+. The amount of K+ in the supernatant was measured using atomic absorption spectrophotometer (AAS, Hitachi Z-5000) and the CEC was calculated.
2.3. Adsorption Experiments
Adsorption of Pb was conducted using 50 mL of Pb(NO3)2 solution with initial concentrations of 0, 0.05, 0.10, 0.15, 0.20, 0.30, 0.45, 0.60 mM and 0.01 g of Na+ saturated zeolite A4, X, Y and mordenite samples. The experiments were conducted in the presence of 100 mM NH4NO3 and varying initial solution pH of 3, 4 and 5. The adjustment of pH was done using 1 mM HNO3 for pH 5 and 4, and 100 mM HNO3 for pH 3 by adding drops of the HNO3 solution to a mixture of Pb(NO3)2 and NH4NO3 in concentrations that have been mentioned earlier. Experiments were conducted at 26˚C ± 0.1˚C in a temperature controlled water bath using centrifuge bottles with aluminum foil covering. Shaking was done for 1 h which was experimentally pre-determined to be sufficient for adsorption equilibrium to be reached. After shaking, the samples were centrifuged at 1600 g for 10 min then pH of the supernatant was measured. Lastly, the concentration of Pb in the supernatant was measured by AAS. The amount of Pb adsorbed was calculated from the difference between initial and final Pb concentra- tions.
3. Results and Discussion
Table 1 indicates the CEC of the samples. Zeolite A4 was noted to be the sample with the highest CEC compared to the rest of the samples. Having a higher CEC was thought to be a property that would contribute positively to its adsorptive performance. The CEC data as presented in Table 1 has also been reported in our previous publication on the adsorption of Cu of different Zeolites [17] .
3.1. Adsorption Isotherms
Table 2 indicates the percentage adsorption of Pb using the studied zeolite samples. At low initial concentration of Pb (0 - 200 µmol∙L−1), Zeolites A4 and Faujasite X were shown to have removed 100% of the Pb from the solution while at the highest initial concentration that was studied (600 µmol∙L−1), the removal percentages were 76% and 69% for zeolites A4 and faujasite X. This means that these samples can be used to clean up both high andvery low concentrations of pollutants, even those that meet the standard of Pb set by the EPA of 0.005 mg∙L−1 for drinking and 0.05 mg∙L−1 for waste water.
Figures 1-4 are the isotherms of Pb adsorption at different initial solution pH ranging from 3 - 5. It was observed that adsorption was highest at an initial pH of 5 among the samples except mordenite whose adsorption
![]()
Table 2. Percentage removal of Pb by Zeolites A4, X, Y and mordenite.
![]()
Figure 1. Pb adsorption isotherms on Zeolite A4 with varying initial pH.
![]()
Figure 2. Pb adsorption isotherms on Faujasite X with varying initial pH.
![]()
Figure 3. Pb adsorption isotherms on Faujasite Y with varying initial pH.
was almost constant across this pH range. The uptake capacity of metal ions and the adsorption mechanism in- volved are known to be dependent on the pH of the solution, which affects the degree of ionization, surface charge of the adsorbent and the speciation of the adsorbate [18] [19] .
The sorption of metal ions decreased with the acidity of the solution. As the pH increased, more negatively
![]()
Figure 4. Pb adsorption isotherms on mordenite with varying initial pH.
charged surfaces became available, therefore leading to a greater metal uptake. It is conceivable that at low pH values, there were more H3O+ ions in the solution, and competition existed between the positively charged hydrogen ions and metal ions for the available adsorption sites on the zeolite surface. As the pH increased, more of the positively charged metal ions in solution were adsorbed on the negative surface and thus the percentage removal of the metal ions increased. In the current experiments, pH 5 was noted to the optimum pH for the adsorption and without visible precipitation of Pb. At initial solution pH 4 and 5, the adsorption isotherm was very steep in the lower concentration range especially for zeolites A4 and faujasite X. This indicated that these samples can effectively remove low concentrations of Pb from polluted environments, which is a property that is hardly achieved by most of the available adsorbents. At the same time, the current experiments were also conducted in the presence of NH4NO3 which might have also been a possible source of positive charge which might have occupied the available negativecharge on the zeolite.
Generally, the adsorption of Pb in comparison with other heavy metals has proven to be favorable for most adsorbents. In our experiments, Pb adsorption on zeolites A4 and Faujasite X has also been higher than that of Cu (2500 and 2000 mmol∙kg−1 respectively for Pb and 1429 and 909 mmol∙kg−1 for Cu). These results are in agreement with the findings of other researchers. In their study on the adsorption of Cu and Pb on raw zeolite, Zou et al. [20] found that adsorption was highest for Pb unlike Cu. The adsorptive capacity of raw zeolite was 61 mmol∙kg−1 for Pb and 134 mmol∙kg−1 for Cu. Lee [21] reported that the adsorption of Pb on Na-P1 was 1286 mmol∙kg−1, followed by Cu, 1156 mmol∙kg−1. Exceptionally high adsorption of Pb was reported by Jha [22] with values of 3165 mmol∙kg−1 for Pb and 2598 mmol∙kg−1 for Cu.
The adsorptive capacity of the samples was analyzed using Langmuir adsorption analysis method. The Langmuir linear equation used is:
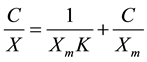
where C is the equilibrium concentration (mmol∙L−1), X the amount adsorbed (mmol∙kg−1), Xm the maximum adsorption (mmol∙kg−1) and K a constant related to binding energy (L∙mmol−1). The results of the Langmuir analysis are as indicated in Table 3.
The immobilization of heavy metal ions from aqueous solutions on zeolite is a complex process consisting of ion exchange and adsorption, likely to be accompanied by precipitation of metal ion hydroxide complexes on active sites of the particle surface [23] [24] . Zeolite A4 was noted to have portrayed the highest adsorptive capacity for Pb in comparison with other zeolite samples. The maximum adsorptive capacities (Xm) of all the samples are as indicated in Table 3. The amount of negative charge on Zeolites A4, Faujasite X, Faujasite Y and Mordenite, which has been occupied by Pb (assuming that Pb in the solution existed as Pb2+ at pH 5) translates to a CEC of 5000, 4000, 1176 and 358 mmol∙kg−1 respectively. These values are lower than the CEC values reported in Table 1. The difference with the CEC is due to the availability of other sources of positive charge other than Pb alone during the adsorption experiment i.e. H+ and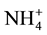 . It is also noticeable that the difference be-
. It is also noticeable that the difference be-
![]()
Table 3. Langmuir parameters for Pb adsorptionat initial pH of 3, 4 and 5.
tween the CEC and the amount of Pb adsorbed is wider for low adsorption samples (Faujasite Y and Mordenite) unlike A4 and Faujasite X. Results from previous experiments conducted using Cu indicated that the amount of negative charge on Zeolites A4, Faujasite X, Faujasite Y and Mordenite occupied by Cu2+ was 2858, 1818, 1334 and 384 mmol∙kg−1 [17] . It follows that the adsorption of Pb is twice as high on Zeolite A4 and Faujasite X than is the same with Cu. The high adsorptive capacity for A4 might be attributed to its high CEC as indicated in Table 1. The binding energy was also high for A4 indicating a strong bond between the adsorbent and adsorbate.
3.2. pH Dependence of Pb Adsorption
The pH value of the solution has a significant impact on the removal of heavy metals, because it determines degree of ionization of metal ions in aqueous solution and the charge on the surface of adsorbents [25] . Table 4 is an indication of the change in pH after adsorption. It was generally noted that the equilibrium pH increased for all samples. Although such an increase was observed, there was no clear sign of precipitation at equilibrium. Preliminary experiments indicated that precipitation of Pb was only noticeable at pH > 6.5. A plot of the amount adsorbed for each sample and the equilibrium pH resulted into a figure that portrays the pH dependence of the samples. Figure 5 is the plot of the amount adsorbed and equilibrium pH at initial concentration of 600 µmol∙L−1. Zeolite A4 and Faujasite X have steep isotherms which are spread over a narrow range of pH compared to Faujasite Y and Modernite whose isotherm is spread over a wider pH range (note that for Zeolite A4 and Faujasite X, an additional data point; equilibrium pH at initial pH of 3.5 was obtained and inserted on Figure 5). It also highlighted the high dependence on pH, of the adsorption of Pb within this pH region. In the pH range of 2 - 6, the sorption percentage increases gradually with increasing pH. Adsorption is mainly dominated by ion exchange as H+ ions are competitive to Pb ions for ion exchange sites. The figure further highlights the fact that the adsorption of Pb is strongly affected by the increasing concentration of proton (H+), in other words, decreasing pH.
Since some amount of proton (HNO3) was added for pH adjustment, Zeolites A4 and Faujasite X, having high proton selectivity, adsorbed most of the available proton onto its surface hence the narrow range of changing pH at equilibrium. Faujasite Y and Mordenite on the other hand, have lower proton selectivity which resulted into more protons being available in the solution at equilibrium. The available protons are the ones that contributed to the wider range of pH change at equilibrium. In their study on the decrease in the CEC of zeolites at neutral pH, Munthali et al. [26] highlighted the pH dependence of Na+-saturated A4 and Na-P1 zeolites. With increasing the amount of HCl addition to the zeolites, equilibrium pH decreased, and CEC or the amount of Na+ retained of the two zeolites decreased. The CEC of Na-P1 type zeolite began to decrease from pH 7, and that of Linde-type A zeolite began to decrease from pH 9. These indicated that the adsorption of Na+ on the two zeolites is pH-dependent. Similarly, Figure 5 shows that the adsorption of Pb on the A4 and Faujasite X is strongly pH-dependent, and the adsorption of proton started from pH around 5.5.
4. Conclusion
The removal of heavy metals is a vital step in reducing the negative effects caused by their presence in non-tar- get environments. Use of low cost sorbents such as zeolite can be considered as a viable option for the treatment of water pollution from heavy metals. In the study, Zeolites A4 and Faujasite X have proven to be substances that offer high adsorption of Pb. They can be used for removing heavy metals even at low levels of pollution. At low initial concentration of Pb, Zeolites A4 and Faujasite X were shown to have removed 100% of the Pb from the solution while at the highest initial concentration that was studied (600 µmol∙L−1), the removal per-
![]()
Table 4. Equilibrium pH of the samples.
![]()
Figure 5. pH dependence of the samples for Pb adsorption at initial Pb concentration of 600 µmol∙L−1 and pH of 5.
centages were 76% and 69% for zeolite A4 and faujasite X. This means that these samples can be used to clean up both high and very low concentrations of pollutants, even those that meet the standard of Pb set by the EPA of 0.005 mg∙L−1 for drinking and 0.05 mg∙L−1 for waste water. However, the fact that the effectiveness of Pb removal by the mentioned zeolites is pH dependent, their application has to be with caution.
NOTES
*Corresponding author.