Hexavalent Chromium Removal and Reduction to Cr (III) by Polystyrene Tris(2-aminoethyl)amine ()
1. Introduction
Chromium exists in the environment in two main oxidation states Cr (III) and Cr (VI) [1] . Cr (III) is an essential element for humans and is much less toxic than Cr (VI), it is required to potentiate insulin and for normal glucose metabolism [2] [3] . Cr (III) is poorly adsorbed by any route so the toxicity of chromium is attributed to the Cr (VI) form [4] . Cr (VI) can be absorbed by the lung and gastrointestinal tract, and even to a certain extent by intact skin. If Cr (VI) is reduced to Cr (III) extracellularly, the toxicity is not observed. Cr (VI) can be reduced intracellularly by hydrogen peroxide, glutathione reductase and ascorbic acid to produce reactive intermediates. Any of these species could attack DNA, proteins and membrane lipids thereby disrupting cellular integrity and functions. Exposure to chromium (VI) can cause respiratory, renal, hepatic, gastrointestinal, cardiovascular and hematological problems. Also Cr (VI) is considered to be carcinogenic [5] [6] . Cr (VI) is introduced into natural waters by a variety of industrial processes including textile, dyes and pigment production, film and photography, galvanometry, leather tanning, electroplating and metal finishing industries [7] . A number of treatment methods for the removal of metal ions from aqueous solutions have been reported. These include reduction, ion exchange, electro dialysis, electrochemical precipitation, evaporation, solvent extraction, reverse osmosis, chemical precipitation and adsorption. Most of these methods have a lot of disadvantages including high operational cost [8] . Recently, adsorption processes utilizing natural low cost adsorbents were employed in order to remove chromium from aqueous solutions. Some of these processes were also able to reduce Cr (VI) to Cr (III) [1] [10] -[13] . On the other hand, specific sorbents consisting of polymer microspheres containing metal chelating ligands were employed for heavy metal ion removal [9] .
Herein, the use of polystyrene tris(2-aminoethyl)amine microspheres as an efficient matrix for the removal of Cr (VI) from aqueous solutions is reported. The effects of contact time, pH and adsorbent dosage on the adsorption efficiency are studied. The maximum adsorption capacity is evaluated using Langmuir adsorption isotherm and compared with reported values.
2. Experimental
2.1. Chemicals and Materials
Polystyrene tris(2-aminoethyl)amine, Potassium dichromate, 1,5-Diphenyl carbazide, sodium hydroxide, and sulfuric acid were purchased from Sigma-Aldrich (Israel) and were used as received. All solutions were prepared in deionized distilled water. Cr (VI) stock solution (1000 mg/L) was prepared by dissolving the appropriate amount of K2Cr2O7 in deionized distilled water. This solution was diluted as required to obtain 5.0 - 80 mg/L Cr (VI) standard solutions. The batch experiments were carried out in 100 ml Erlenmeyer flasks by agitating a pre-weighed amount of the polymer with 50 ml of the aqueous Cr (VI) solution at 25˚C ± 1˚C. The initial pH of the solution was adjusted using 0.2 N H2SO4. A muli-element standard solution (51844) for calibration of the ICP-MS was obtained from Sigma-Aldrich (Israel).
2.2. Analysis of Adsorbate and Adsorbent
The concentration of Cr (VI) was determined colorimetrically using a Perkin-Elmer Lambda 5 UV-Visible spectrophotometer and the total Chromium concentration was determined using inductively coupled plasma-mass spectrometer (ICP-MS, Agilent Technologies 7500 Series). A calibration curve for Cr (VI) was obtained by plotting the absorbance of Cr (VI) solutions of different concentrations after complexation with the 1,5-di- phenyl carbazide ligand at 540 nm. The curve was linear within the selected Cr (VI) concentrations with a correlation coefficient (R2) of 0.9996. While a second calibration curve for total Cr was prepared by analyzing the standard solutions prepared from a multi-element standard solution by ICP-MS. The curve was linear through the selected concentrations with a correlation coefficient (R2) of 0.9987.
The adsorbent dosage was varied in the range of 2.00 and 30.0 g/L with initial Cr (VI) concentration of 10.0 mg/L. Samples were analyzed for total Cr (ICP) and Cr (VI) (UV-Vis) concentrations after a contact time of 180 minutes and at pH = 5.0.
Scanning electron microscopy (SEM) and (EDS) analyses of the adsorbent before and after complexation with chromium ions were recorded on JEOL model, JSM-5410 LV-country. The samples were mounted on metal stubs and then coated with gold (Polaron Spatter coater). The images were taken with an accelerating voltage of 25 kV, at high vacuum (HV mode) and secondary electron image (SEI). The analysis was done using Oxford systems-Liquid Nitrogen cooled solid state Energy Dispersive Spectrometer detector and link ISIS software.
Regeneration of the polystyrene tris(2-aminoethyl)amine was performed by mixing the chromium-chelated adsorbent with 1 M KCl aqueous solution and then agitated for 2 hours. The regenerated adsorbent was tested by agitating it with 10 ppm Cr (VI) solution for 2 hours and the percent removal was calculated by determining the initial and the final concentration of Cr (VI) in the solution using the colorimetric method.
3. Results and Discussion
3.1. Effect of Adsorbent Dosage
Figure 1 and Figure 2 summarize the effect of adsorbent dosage on the percent removal of Cr (VI) and total Cr, respectively. Inspection of these figures reveals that the percent removal of Cr (VI) and of total Cr increased with increasing adsorbent dosage and attained a constant value after a dosage of 10 g/L. Hence the optimum dosage for the removal of Cr (VI) by polystyrene tris(2-aminoethyl)amine was set at 10.0 g/L.
3.2. Effect of Contact Time
The effect of contact time on the percent removal of Cr (VI) and on the concentration of total Cr by polystyrene tris-(2-aminoethyl)amine with the optimum dosage of 10 g/L is demonstrated in Figure 3 and Figure 4. The
![]()
Figure 1. Percent removal of Cr (VI) on polystyrene tris(2-aminoethyl) amine as a function of adsorbent dosage as determined by UV-Visible spectroscopy (T = 25˚C, Contact time = 3 hours, pH = 5, Initial concentration = 10 ppm).
![]()
Figure 2. Percent removal of total Cr on polystyrene tris(2-aminoethyl) amine as a function of adsorbent dosage as determined by ICP-MS spectrometry (T = 25˚C, Contact time = 3 hours, pH = 5, Initial concentration = 10 ppm).
![]()
Figure 3. Percent removal of Cr (VI) on polystyrene tris(2-aminoethyl) amine as a function of contact time by UV-Visible spectroscopy (T = 25˚C, pH = 5, Adsorbent dosage = 10 g/L, Initial concentration of Cr (VI) = 10 ppm).
![]()
Figure 4. Percent removal of total Cr on polystyrene tris(2-aminoethyl) amine as a function of contact time by ICP-MS spectrometry (T = 25˚C, pH = 5, Adsorbent dosage = 10 g/L, Initial concentration of Cr (VI) = 10 ppm).
percent removal as measured by Cr (VI) and by total Cr increased with increasing contact time and attained equilibrium at 120 minutes. These results revealed that 120 minutes was the optimum contact time needed to attain equilibrium. Therefore, in all subsequent measurements, the adsorption contact time was set at 120 minutes.
Figure 5 displays the effect of contact time on the emergence of Cr (III) in solution. It is evident that the concentration of Cr (III) in solution passes through an induction period, an adsorption period and a saturation period. It took 20 min for Cr (III) to build up in solution. Once accumulated, it is removed by adsorption on the polymer until it reached saturation. This explains the observed fluctuation in its concentration as function of time.
3.3. Effect of pH
The percent removal of Cr (VI) was studied at different pH values ranging between 2.0 - 6.0 (Figure 6). Whereas the percent removal of Cr (VI) stayed almost constant as the pH was increased, that of the total Cr was found to vary significantly with pH (Figure 7). This led to the conclusion that the difference was due to the emergence of Cr (III) ions in the solution. Apparently some of the bound Cr (VI) ions were reduced by the adsorbent to Cr (III) ions. The concentration of the latter was determined as the difference between the total chromium obtained by ICP-MS and Cr (VI) obtained by UV-Vis spectroscopy. Inspection of Figure 7 reveals that at
![]()
Figure 5. Concentration of Cr (III) found in solution as a function of contact time with polystyrene tris(2-aminoethyl)amine by ICP-MS spectrometry (T = 25˚C, pH = 5, Adsorbent dosage = 10 g/L, Initial concentration of Cr (VI) = 10 ppm).
![]()
Figure 6. Percent removal of Cr (VI) on polystyrene tris(2-aminoethyl) amine as a function of pH by UV-Visible spectroscopy (T = 25˚C, Adsorbent dosage = 10 g/L, Contact time = 2 hours, Initial concentration = 10 ppm).
![]()
Figure 7. Concentration of Chromium remaining in solution as a function of pH (T = 25˚C, Contact time = 2 hours, Adsorbent dosage = 10 g/L, Initial concentration = 10 ppm).
pH = 2 the concentration of Cr (III) available in solution was about 7 ppm, then it dropped significantly when the pH was raised. It is known that the reduction of Cr (VI) to Cr (III) is also catalyzed by H+ [10] [11] . Hence the rate of reduction is much higher at pH = 2 than that at pH 6, leading to the observed increase in [Cr (III)] at pH = 2.
In agreement with previously reported results [10] [11] , two mechanisms appear to be involved in the removal of Cr (VI) by polystyrene tris-2-(aminoethyl) amine; adsorption and reduction. So the removal can be performed by any of the two processes. If the intention is to remove Cr (VI), then pH = 6 should be chosen. While if the intention is to detoxify Cr (VI), then the redox process is the preferred route and this should be performed at pH = 2.
3.4. Effect of Initial Cr (VI) Concentration
The effect of initial Cr (VI) concentration on the percent removal of Chromium was studied over the range 10.0 to 80.0 mg/L at the optimum contact time of 120 minutes, pH = 5.0 and at an adsorbent dosage of 10 g/L (Figure 8). The results obtained by UV-Visible and by ICP-MS, indicated that the percent removal of Cr (VI) decreased with increasing initial concentration.
3.5. SEM and EDS Analyses
The scanning electron microscope images (Figure 9) showed an increase in average particle size from 71 µm (polystyrene tris(2-aminoethyl)amine alone) to 88 µm (polystyrene tris(2-aminoethyl)amine bound to Cr (VI)), this increase in average particle size is caused by the complexation of Cr (VI) to the polymer.
![]()
Figure 8. Percent removal of Cr (VI) and total Cr on polystyrene tris(2-aminoethyl)amine as a function of initial concentration of Cr (VI) (T = 25˚C, pH = 5, Contact time = 2 hours, Adsorbent dosage = 10 g/L).
![]() (a) (b)
(a) (b)
Figure 9. SEM images for microspheres of (a) polystyrene tris(2-ami- noethyl) amine (b) polystyrene tris(2-aminoethyl)amine bound to Cr (VI).
Elemental analysis using Energy Dispersive Spectrometry showed the appearance of Cr after the complexation of polystyrene tris(2-aminoethyl)amine with Cr (VI) ions. The appearance of S is attributed to the use of sulfuric acid in pH adjustment (Table 1 and Table 2).
3.6. Regeneration of the Adsorbent
The concentration of Cr (VI) desorbed was 0.27 ppm while the total chromium found in the solution was 4.13 ppm. The difference (3.85 ppm) represented the concentration of Cr (III) exchanged by KCl solution. Hence, we can conclude that the adsorbent reduced almost all the bound Cr (VI) to Cr (III). The percent desorption was calculated using the initial concentration of Cr (VI) on the adsorbent and the final concentration of Cr (III) and Cr (VI) in KCl solution and was found to be 89.3%.
3.7. Testing the Removal of Cr (VI) by the Regenerated Adsorbent
The percent removal of Cr (VI) by the regenerated adsorbent was 95.11%. The removal capacity of the latter adsorbent was comparable to that of the original polymer which was 96.74%. This demonstrates the high efficiency of KCl in desorbing chromium from polystyrene tris(2-aminoethyl)amine.
3.8. Adsorption Isotherms
To model the adsorption behavior, two adsorption isotherms were studied and their correlation with the experimental data was assessed.
3.8.1. Langmuir Isotherm
Langmuir equation may be written as
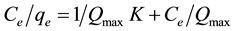
where qe is the amount of solute adsorbed per unit weight of adsorbent (mg/g), Ce is the equilibrium concentration of solute in the bulk solution (mg/L), Qmax is the adsorption capacity (mg/g), K is the constant related to the free adsorption energy.
A plot of Ce/qe versus Ce was linear and the constants Qmax and K were determined from the slope and intercept of the plot respectively. The correlation coefficient obtained with the Langmuir equation was high by UV-Vis (R2 = 0.9997) and ICP-MS (R2 = 0.9891) indicating a good fit between the parameters (Figure 10 and Figure 11). Qmax = 312.27 mg/g and K = 0.947 at T = 25˚C and pH = 5 with 10 g/L adsorbent dosage. Compared with other adsorbents (Table 3), polystyrene tris(2-aminoethyl)amine showed high adsorption capacity.
![]()
Table 1. Elemental analysis of polystyrene tris(2-aminoethyl)amine.
![]()
Table 2. Elemental analysis of polystyrene tris(2-aminoethyl)amine bound to Cr (VI).
![]()
Table 3. Adsorption capacity of various adsorbents.
![]()
Figure 10. Langmiur isotherm for the adsorption of Cr (VI) using UV- Vis spectroscopy (T = 25˚C, pH = 5, Contact time = 2 hours, Adsorbent dosage = 10 g/L).
3.8.2. Freundlich Isotherm
The Freundlich isotherm is expressed by the following equation
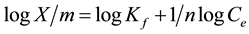
where X/m is the amount of solute adsorbed per unit weight of adsorbent (mg/g), Ce is the equilibrium concentration of solute in the bulk solution (mg/L), Kf is a constant indicative of the relative adsorption capacity of the adsorbent (mg/g), 1/n indicates the intesity of the adsorption.
A plot of logX/m versus logCe was non linear, indicating bad fit between parameters (Figure 12). The regression coefficient (R2) were 0.9997 for Langmuir isotherm and 0.8837 for Freundlich isotherm. The R2 values indicated that Langmuir isotherm model is the best to describe the removal of Cr (VI) ions by polystyrene tris(2-aminoethyl)amine. The Freundlich isotherm model does not agree well with the adsorption process. So monolayer coverage occurred on the available active sites on the adsorbent.
3.9. Proposed Mechanism of Adsorption
Apparently, the adsorption mechanism initially starts with an electrostatic attraction between the ammonium ions, generated at acidic pH from the amino groups attached to the polymer, with the predominant chromium
![]()
Figure 11. Langmiur isotherm for the adsorption of Cr (VI) using ICP- MS spectroscopy (T = 25˚C, pH = 5, Contact time = 2 hours, Adsorbent dosage = 10 g/L).
![]()
Figure 12. Freundlich isotherm for the adsorption of Cr (VI) using UV- Vis spectroscopy (T = 25˚C, pH = 5, Contact time = 2 hours, Adsorbent dosage = 10 g/L).
species, ![]() [16] . This is followed by reduction to Cr (III) and complex formation with the four amino groups situated on the polymer [23] [24] . The adsorbent itself being oxidized to the N-oxide as apparent from the IR spectrum that shows a small band at about 940 cm−1 corresponding to the N-O stretching vibration. Also the bands in the region 1100 - 800 cm−1 corresponding to C-N stretching vibrations have shifted to lower frequencies indicating complexation of the polymer with chromium (Figure 13). The generated Cr (III) was either released to the aqueous phase or adsorbed depending on the availability of free sites. Upon addition of 1 M KCl to the adsorbent, Cr (III) was released to the solution and was determined by ICPMS (Figure 14).
[16] . This is followed by reduction to Cr (III) and complex formation with the four amino groups situated on the polymer [23] [24] . The adsorbent itself being oxidized to the N-oxide as apparent from the IR spectrum that shows a small band at about 940 cm−1 corresponding to the N-O stretching vibration. Also the bands in the region 1100 - 800 cm−1 corresponding to C-N stretching vibrations have shifted to lower frequencies indicating complexation of the polymer with chromium (Figure 13). The generated Cr (III) was either released to the aqueous phase or adsorbed depending on the availability of free sites. Upon addition of 1 M KCl to the adsorbent, Cr (III) was released to the solution and was determined by ICPMS (Figure 14).
![]()
Figure 13. FTIR of (a) polystyrene tris(2-aminoethyl)amine and (b) polystyrene tris(2-aminoethyl)amine-Cr complex.
![]()
Figure 14. Proposed mechanism of adsorption of Cr (VI) by polystyrene tris(2-aminoethyl)amine.
3.10. Adsorption Capacity of Various Adsorbents
The adsorption capacity of the polystyrene tris(2-aminoethyl)amine under ambient pH and at 25˚C was found to be 312.27 mg/g of adsorbent. This is about five times more than the most efficient adsorbent reported earlier, particularly, Neem sawdust and cationized cellulose (Table 3). The acceptable cost and the efficiency of our adsorbent makes it a good candidate for the removal of Cr (VI) from aqueous media.
4. Conclusion
Polystyrene tris-2-(aminoethyl)amine has been successfully used to remove Cr (VI) from aqueous solutions. The removal of Cr (VI) was best at pH 6, and the percentage of Cr (VI) removed depended on adsorbent dosage, contact time and initial Cr (VI) concentration. During the adsorption process detoxification of Cr (VI) occured by its reduction to Cr (III). The Langmuir isotherm model agreed well with the experimental data and the maximum adsorption capacity was 312.27 mg/g.
Acknowledgements
We wish to thank Professor Dr. Z. Abdeen for his valuable financial support which made this work possible. We also wish to thank the Aquatic and Aquaculture Research Laboratory at Al-Quds University for performing the ICP-MS analysis.
NOTES
*Corresponding author.