Experimental Study and Thermodynamic Analysis of High Temperature Interactions between Boron Carbide and Liquid Metals ()
1. Introduction
The wetting behavior of molten metals or alloys in contact with ceramic substrates is of crucial importance in a variety of processes, such as the fabrication of metal-ceramic composites (MCCs), the infiltration of ceramic preforms by molten metals, and metal/ceramic or ceramic/ceramic joining. It is not surprising, therefore, that extensive efforts have been devoted to clarifying the underlying factors that determine the wetting behavior and properties of the ceramic-liquid metal interface.
Among the non-oxide ceramics, boron carbide stands out by virtue of its technological importance ensuing from its mechanical properties, elevated thermal conductivity, and thermal shock and oxidation resistance. Bo- ron carbide and boron carbide-based cermets are promising materials for a variety of applications that require elevated hardness, good wear, and corrosion resistance. In fact, many attempts were made to fabricate compo- sites based on boron carbide and aluminum [1] - [3] . Aluminum was the most common metal chosen due to its low density and low melting temperature, which make it suitable for pressure-free infiltration processes. Never- theless, improved wetting and successful infiltration were achieved above 1200˚C, which introduces undesired effects. Reactions at the ceramic/metal interface cause the formation of undesired brittle phase, including Al4C3, which is hygroscopic and drastically deteriorates the mechanical properties of the composite. Wetting properties of boron carbide and Ni-based brazing alloys were also studied due to the interest in Ni-based filler metals for high temperature applications [4] [5] . It could be stated that generally ceramics are partially wetted (contact an- gle, 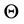 , is much greater than 90˚) unless a chemical reaction occurs at the ceramic/molten drop interface. The generic reactions are dissolution of the ceramic substrate in the melt or the formation of new phases at the inter- face [6] - [8] , which could promote spreading remarkably.
, is much greater than 90˚) unless a chemical reaction occurs at the ceramic/molten drop interface. The generic reactions are dissolution of the ceramic substrate in the melt or the formation of new phases at the inter- face [6] - [8] , which could promote spreading remarkably.
B4C is unique with respect to wetting since it belongs to both the carbides and borides. Carbides can be gen- erally divided to two groups: the first is the narrow composition range carbides such as SiC and Al4C3, the second group is carbides such as TiCx and ZrCx 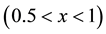 with a wide composition range. Silicon carbide’s wetting properties were extensively investigated because of its use as a structural material in high temperature applications such as reinforcement in metal-matrix composites [9] .
with a wide composition range. Silicon carbide’s wetting properties were extensively investigated because of its use as a structural material in high temperature applications such as reinforcement in metal-matrix composites [9] .
Marin et al. [10] showed that that pure Cu poorly wets SiC (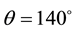 at 1100˚C) due to large Si dissolution in molten Cu. The addition of Si to Cu inhibits the Cu-SiC interface reaction and leads to improved spreading of the SiC substrate (
at 1100˚C) due to large Si dissolution in molten Cu. The addition of Si to Cu inhibits the Cu-SiC interface reaction and leads to improved spreading of the SiC substrate ( , XSi » 0.15). The spreading mechanism was not explained but an attempt towards un- derstanding closely related phenomena was made by Rado et al. [11] , who investigated the wetting properties of the Ag and SiC/(Ag-Si) systems. They found that a silver-rich Ag-Si alloy doesn’t react with SiC but wets it fairly well (40˚ contact angle). They suggested that this behavior is not due to adsorption of Si at the liquid met- al/SiC interface but rather to direct bonding between Ag and SiC, implying that chemical bonds exist at the in- terface.
, XSi » 0.15). The spreading mechanism was not explained but an attempt towards un- derstanding closely related phenomena was made by Rado et al. [11] , who investigated the wetting properties of the Ag and SiC/(Ag-Si) systems. They found that a silver-rich Ag-Si alloy doesn’t react with SiC but wets it fairly well (40˚ contact angle). They suggested that this behavior is not due to adsorption of Si at the liquid met- al/SiC interface but rather to direct bonding between Ag and SiC, implying that chemical bonds exist at the in- terface.
The role of stoichiometry is well established in ceramics that display a relatively wide composition range, such as TiC. The study of the TiC/(Cu-Ti, Al) system illustrates the influence of stoichiometry on the wetting behavior [7] [12] . The key factor that controls the interface reaction is the activity of the metal in the carbide. In the case of the stoichiometric carbide (TiC), Cu is able to dissolve negligible quantities of Ti (~10−6 atom frac- tion at 1400 K), which explains the high contact angle obtained in the TiC/Cu (~130˚) system. The ability to dissolve relatively large quantities of Ti in contact with non-stoichiometric TiCx improves spreading remarkably (~20˚ in the Ti2C/Cu system). In practice, the morphology and composition in the vicinity of the interface influ- ences spreading as well as the departure from thermodynamic equilibrium. For example, a metal like Au with high affinity for Ti in contact with TiC might cross the graphite-containing region, inducing graphite precipita- tions that are not wetted by the non-carbide forming metals such as Au or Cu.
The wetting properties of borides are much less known and investigated compared to carbides. Kharlamov et al. [13] studied the wetting properties of hot-pressed aluminum borides by molten Al and Cu. Liquid Al wets all Al-borides.
Muolo et al. [14] [15] studied the wetting behavior in the ZrB2/Ag, Cu, eutectic Ag-Cu, and eutectic Ag-Cu-2 wt% Zr systems as well in the TiB2/Cu, Ag-Cu systems. Additions of 2 wt% Zr to Ag and to the eutectic Ag-Cu alloy improved wetting remarkably (~15˚ and ~50˚, respectively). This behavior was attributed to the adsorbed Zr at the ZrB2/melt-interface thereby increasing its metallic character. The unique wetting behavior of TiB2 in contact with pure Cu and Au was also reported in [16] , where the measured contact angles are (~55˚ and ~15˚, respectively) although only minor quantities of Ti could be dissolved in liquid Cu and Au at 1423 K (~10−10 and ~10−7 atomic fractions, respectively) and no interface interaction was detected.
Boron carbide, unlike metallic borides, is highly covalent and displays a wide composition between B10.5C and B4C (8.8 - 20 at%C) [17] [18] . Regarding chemical interaction with boron carbide, metals can be divided to two groups. Reactive metals, such as Ti, Si, Al, Fe, Ni, can readily react with boron carbide and form new car- bide or boride phases. Boron carbide could easily react with these metals due to relatively low thermodynamic stability compared to other carbides (the reported standard enthalpy of formation of boron carbide is in the range of ?38.9 - 71.5 kJ/mol boron carbide [19] ). The second group contains the so-called non-reactive metals, such as Cu, Au, Ag, and Sn, whose interaction with boron carbide leads to limited carbide dissolution or decomposition, without new phase formation as was established by Naidich [20] (the contact angles of 130˚ - 140˚ were meas- ured between the non-reactive metals and B4C). Nevertheless, the wetting behavior and properties of the B4C/non- reactive metal systems have not been the subject of many studies and satisfactory understanding of these systems doesn’t exist.
It is known that the wetting of non-oxide ceramics by non-reactive metals could be improved by addition of a reactive component that may significantly alter the metal-ceramic interface. In the case of boron carbide, as will be demonstrated in this article, the alloying element could react with C and form a carbide phase, for instance SiC if Si was added. In this case the boron carbide composition changes and the compound composition changes towards higher boron content, BxC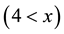 , where
, where 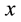 depends on the Si content in the melt. If Ti was added to the non-reactive metal, boride phase formation and graphite precipitation take place. It must be noted that car- bon solubility in these metals is also extremely low (a few ppm) and carbon precipitation during the interaction may take place. The wide composition range of boron carbide offers an additional degree of freedom that could be used to improve wetting. In the present investigation the interface interaction between boron carbide and liq- uid alloys based on the non-reactive metals (pure Cu, Sn, Au, and Ag and selected alloys containing Si, Ti, and Al) and the wetting phenomena in these systems were studied in order to establish the technological parameters required for producing cermets based on boron carbide.
depends on the Si content in the melt. If Ti was added to the non-reactive metal, boride phase formation and graphite precipitation take place. It must be noted that car- bon solubility in these metals is also extremely low (a few ppm) and carbon precipitation during the interaction may take place. The wide composition range of boron carbide offers an additional degree of freedom that could be used to improve wetting. In the present investigation the interface interaction between boron carbide and liq- uid alloys based on the non-reactive metals (pure Cu, Sn, Au, and Ag and selected alloys containing Si, Ti, and Al) and the wetting phenomena in these systems were studied in order to establish the technological parameters required for producing cermets based on boron carbide.
2. Experimental
B4C ceramic samples of a near-theoretical density were obtained from hot-pressed powder (Starck) with 1 - 3 μm particle size. The substrate surface for the wetting experiments were polished to the 0.25 μm diamond paste level and gently cleaned. Sessile drop experiments were performed at 1423 K in a vacuum furnace (10−5 torr) [12] . Alloys were prepared in situ or by an arc furnace, using the appropriate quantities of the relevant elements. Contact angles were measured directly from the magnified profile digital images of the molten metal drop. The solidified drops were cross-sectioned and polished down to 1 μm using SiC papers and diamond pastes. The metal-ceramic interface structure and chemical composition were studied using SEM-EDS (energy dispersive spectrometry) and SEM-WDS (wavelength dispersive spectrometry) techniques. X-ray diffraction was used to characterize the composition of the B4C substrate. Surface analysis (Auger and XPS) is used to study the com- position at the vicinity of the metal/B4C interface.
3. Results
The results are presented in the following order. First the thermodynamic analysis of the system is shown, then spreading and interface characterization results are presented and finally a discussion links the two parts.
Although boron carbide is a covalent compound with a very high melting temperature, it isn’t stable in con- tact with various elements such as Al, Si, Fe or even Cu which tends to dissolve boron. The following paragraph describes the thermodynamic analysis of the ternary B-C-Cu system followed by the four element B-C-Cu-Si system. Other B-C-Me-Si systems were also investigated. The same approach was used to investigate the B-C- Me-Ti system and the B-C-Me (only for Cu and Sn)-Al system and is shown for each studied system.
3.1. The Boron Carbide-Cu and Cu-B Systems
3.1.1. Thermodynamic Analysis
According to the Cu-C and Cu-B phase diagrams, the solubility of carbon in liquid copper at 1200˚C is very low (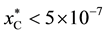 atomic fraction [21] ) and no carbide phase is present in this system. The Cu-B system displays a eutectic reaction at 13.3 at% and 1013˚C. The boron solubility in liquid Cu at 1200˚C (1473 K) is about 25 at%. [22] . Since no stable ternary phase in the Cu-B-C system has been reported, an isothermal section for this system may be constructed, as shown in Figure 1. The phase equilibrium regions are denoted by Roman numerals: I-single phase region of the Cu-B-C liquid solution (L.S.); II-two-phase region of L.S. with boron carbide of various compositions; III-two-phase region of L.S. + graphite; IV-three-phase region with L.S., corresponding to Point O1, + graphite + B4C; V-three-phase region of L.S., corresponding to Point O2, + B10C + boron, saturated with Cu; VI-two-phase region with L.S., corresponding to Point O2, + boron, saturated with Cu. Point O1 cor- responds to the three-phase equilibrium of the liquid solution
atomic fraction [21] ) and no carbide phase is present in this system. The Cu-B system displays a eutectic reaction at 13.3 at% and 1013˚C. The boron solubility in liquid Cu at 1200˚C (1473 K) is about 25 at%. [22] . Since no stable ternary phase in the Cu-B-C system has been reported, an isothermal section for this system may be constructed, as shown in Figure 1. The phase equilibrium regions are denoted by Roman numerals: I-single phase region of the Cu-B-C liquid solution (L.S.); II-two-phase region of L.S. with boron carbide of various compositions; III-two-phase region of L.S. + graphite; IV-three-phase region with L.S., corresponding to Point O1, + graphite + B4C; V-three-phase region of L.S., corresponding to Point O2, + B10C + boron, saturated with Cu; VI-two-phase region with L.S., corresponding to Point O2, + boron, saturated with Cu. Point O1 cor- responds to the three-phase equilibrium of the liquid solution 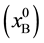 with graphite and stoichiometric boron carbide (B4C). Point O2 corresponds to the three-phase equilibrium of the liquid solution, saturated with B
with graphite and stoichiometric boron carbide (B4C). Point O2 corresponds to the three-phase equilibrium of the liquid solution, saturated with B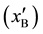 , boron carbide of its lowest carbon content (B10C), and the Cu-B solution. The coordinates of the curve O1-O2 that represents the boundary between single phase and two-phase regions were calculated in detail in [23] . Line 1 (Figure 1), linking unalloyed liquid Cu with stoichiometric B4C, crosses the graphite-containing regions. Therefore, in the course of the metal-ceramic interaction graphite precipitation occurs. However, any line, such as Line 2 that links a liquid Cu-B solution having a boron content greater than 2.4 at%
, boron carbide of its lowest carbon content (B10C), and the Cu-B solution. The coordinates of the curve O1-O2 that represents the boundary between single phase and two-phase regions were calculated in detail in [23] . Line 1 (Figure 1), linking unalloyed liquid Cu with stoichiometric B4C, crosses the graphite-containing regions. Therefore, in the course of the metal-ceramic interaction graphite precipitation occurs. However, any line, such as Line 2 that links a liquid Cu-B solution having a boron content greater than 2.4 at%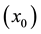 , with stoichiometric boron carbide, does not cross the graphite-containing regions and graphite precipitation during the metal- ceramic interaction is avoided. In this latter case, however, if the overall composition of the reacting system corresponds to Point A within the two phase region, the composition of the phases present (melt and carbide) must change according to the end-points of the tie-line (Line 3) that passes through Point A. Careful examination of the figure shows that the boron content of the liquid will decrease
, with stoichiometric boron carbide, does not cross the graphite-containing regions and graphite precipitation during the metal- ceramic interaction is avoided. In this latter case, however, if the overall composition of the reacting system corresponds to Point A within the two phase region, the composition of the phases present (melt and carbide) must change according to the end-points of the tie-line (Line 3) that passes through Point A. Careful examination of the figure shows that the boron content of the liquid will decrease 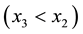 while the carbide will shift to- wards areas of higher boron composition. These changes may be due to boron carbide decomposition in the melt at a B/C ratio less than 4/1 or to boron diffusion from the melt into the near surface layer of the substrate.
while the carbide will shift to- wards areas of higher boron composition. These changes may be due to boron carbide decomposition in the melt at a B/C ratio less than 4/1 or to boron diffusion from the melt into the near surface layer of the substrate.
3.1.2. Spreading and Interface Characterization
A high contact angle close to 110˚ was observed for Cu on B4C substrate at 1423 K, after 30 min contact (Fig- ure 2(a)). As the result of the interaction of molten copper with the substrate, carbon is released from the car- bide and forms a very thin surface layer located at the initial substrate-metal interface. Below that layer, a crater forms, and as it deepens additional carbon is released to form agglomerates dispersed in the molten metal con- tained within the volume of the crater (Figure 2(b)). It has also been established that boron addition to liquid Cu prevents the formation of a crater and leads to improved spreading.
It has been established that boron addition (above 2.8 at%) to liquid Cu prevents the formation of a crater and leads to improved wetting (Figure 3) [24] . Figure 4 shows that experimental results are in good agreement with the thermodynamic calculations shown above. The contact angle observed is the macroscopic contact angle. Pure Cu in contact with B4C dissolves the substrate (Figure 2) and destroys the flat interface that is mandatory for the validity of the Young-Dupré equation [25] [26] and to the classical meaning of the contact angle. There- fore this result was marked differently.
![]()
Figure 1. Schematic isothermal section of the ternary Cu-B-C phase diagram at 1473 K (the Cu-rich corner is out of scale).
![]()
Figure 2. SEM micrographs of the interface after 30 min. contact at 1423 K. (a) High contact angle is clearly visible. A sharp and straight boundary cor- responds to the initial ceramic-metal interface. The boundary separates the bulk of the molten drop from the molten metal in the crater; (b) The B4C/Cu interface, at higher magnification. Cu-B eutectic was formed during solidification.
![]()
Figure 3. The interface between boron carbide and a Cu-B 2.8 at% alloy shows a flat interface, without boron dissolution and graphite precipitation.
![]()
Figure 4. Sessile drop spreading results at 1423 K in the B4C/Cu-B system.
3.1.3. Discussion
In spite of the commonly accepted non-reactive and non-wetting behavior of boron carbide by molten copper, the latter attacks boron carbide substrates forming a crater below the contact area. Thermodynamic analysis of the Cu-B-C system in the Cu-rich corner suggests that boron additions to molten Cu should eliminate the disrup- tion of the boron carbide. The formation of the crater in the boron carbide substrate may be attributed to the dis- sociation of the carbide in contact with unalloyed copper. Due to differences between the specific weight of Cu and that of carbon, the carbon released from the carbide floats upward in the melt and forms agglomerates. Bo- ron that is released dissolves in the liquid Cu. In contrast, the interface between boron carbide and copper alloys with about 3 at% B remained smooth and no evidence of the graphite precipitation was found. This case evi- dently corresponds to the dashed line 2 in Figure 1. The altering of the boron carbide composition in the near- surface region close to the interface with liquid Cu-B melt provides additional confirmation of the validity of the thermodynamic analysis. Similar interface phenomena were observed in the TiCx-Cu system, in which changes of the substrate composition, within the stoichiometric range of the titanium carbide phase, and the wetting be- havior were affected by Ti additions to liquid Cu [27] .
3.2. The Boron Carbide/(Me-Si, Me = Cu, Au, Sn, Ag) Systems
The boron carbide/(Me-Si) study is divided into two parts. The B4C/(Cu-Si) system, due the strong boron disso- lution in liquid Cu at 1423 K, is more complicated than the other Me-Si systems. Therefore the study is focused on this system, and the other systems, which are less complex, broaden the understanding of the interactions in the B4C/(non-reactive metals-Si) systems.
3.2.1. Thermodynamic Analysis of the B-C-Si and the B-C-Si-Me (Cu, Au, Sn) Systems
A calculated isothermal section of the Si-B-C system at 1400 K has been published by Seifert [28] (Figure 5). Over its high carbon content range, namely BxC with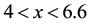 , boron carbide is in two-phase equilibrium with SiC or in three-phase equilibrium with Si and SiC. Only at lower carbon content does silicon boride SiB3 appear and coexist with boron carbide B6.6C. Since copper does not form compounds with either carbon or boron, it may be considered as a diluting agent of silicon, which decreases the silicon activity. In the case of the Cu-B-C-Si system, the reaction between boron carbide and the dissolved Si in molten copper may be expressed as:
, boron carbide is in two-phase equilibrium with SiC or in three-phase equilibrium with Si and SiC. Only at lower carbon content does silicon boride SiB3 appear and coexist with boron carbide B6.6C. Since copper does not form compounds with either carbon or boron, it may be considered as a diluting agent of silicon, which decreases the silicon activity. In the case of the Cu-B-C-Si system, the reaction between boron carbide and the dissolved Si in molten copper may be expressed as:
![]() (1)
(1)
A detailed thermodynamic of the quaternary Cu-Si-C-B system was performed in [23] . Although the expres-
![]()
Figure 5. Isothermal section of the ternary B-C-Si phase diagram at 1400 K [28] .
sion describes the actual reaction, it is not possible to derive the equilibrium parameters because the standard formation energies for B4C1−x are unknown. In order to overcome this obstacle a different approach was used. For a thermodynamic analysis of SiC being formed by carbon that originated in non-stoichiometric boron car- bide and SiB3 being formed from B and Si dissolved in liquid Cu, the chemical reactions considered are:
![]() (2)
(2)
![]() (3)
(3)
where the parentheses signify that Si and B are in the liquid solution and the brackets that carbon is in the B-C solid solution, within the composition limits corresponding to the stability range of the boron carbide phase. The thermodynamic properties of the melt were treated by the Redlich-Kister approach [29] . According to this ap- proach the thermodynamic properties of the multi-component liquid solution could be expressed by taking into account the thermodynamic properties of the binary solutions (expressed by the interaction parameters![]() ,
,![]() ). The different binary systems, the coefficients
). The different binary systems, the coefficients ![]() and
and![]() , were derived at 1423 K and are summarized in Table 1.
, were derived at 1423 K and are summarized in Table 1.
Figure 6 shows the equilibrium results. Each point on the curve corresponds to a certain boron carbide com- position ![]() in equilibrium with the liquid Me-Si-B solution at a definite composition of the melt. The area above the curve corresponds to the two-phase region consisting of boron carbide of various compositions with the liquid solution. The region below the curve corresponds to the three phase equilibrium: Boron carbide of various compositions, SiC, and the liquid solution. The coordinates of the invariant Point A determine the para-
in equilibrium with the liquid Me-Si-B solution at a definite composition of the melt. The area above the curve corresponds to the two-phase region consisting of boron carbide of various compositions with the liquid solution. The region below the curve corresponds to the three phase equilibrium: Boron carbide of various compositions, SiC, and the liquid solution. The coordinates of the invariant Point A determine the para-
![]() (a)
(a)![]()
![]() (b) (c)
(b) (c)
Figure 6. Phase equilibria in the Me-boron carbide-silicon melts (Me = Cu, Au, Sn) at 1423K. Cu-boron carbide-Si (a). Au- boron carbide-Si (b); Sn-boron carbide-Si (c).
meters of the four phase equilibrium where, in addition to the phases mentioned above, silicon boride (SiB3) appears. The limit composition of the boron carbide phase that may be in equilibrium with the melt and SiC corresponds to ~B6.5C. This value is in good agreement with the composition of boron carbide derived from the reported data for ternary Si-B-C phase diagram [28] (Figure 5). According to the calculated diagrams (Figure 6), the Me-Si alloys that are in equilibrium with B4C and SiC contain 11 at% Si for the B4C/Cu-Si, 10 at% Si for the B4C/Au-Si system, and 0.03 at% Si for the B4C/Sn-Si system, which reflect the departure from ideality (negative or positive) of the various liquid solutions.
3.2.2. Spreading and Interface Characterization
The equilibrium contact angles at 1423 K as a function of the alloying element composition are shown in Figure 7.
The interface characterization in the (Sn-Si)/B4C is shown in Figure 8. A very thin (less than 1 μm) conti- nuous SiC layer was detected by WDS analysis at the interface. In this case, the presence of free carbon is avoided; the extremely thin SiC layer formed on the substrate is wetted by the (Sn-Si) molten alloy. At the in- terface of boron carbide with Cu-Si alloys containing less than 13 at% Si, the presence of a small crater with a thin discontinuous layer on its top was observed (Figure 9). According to X-ray mapping of C, this region con- sists of very fine graphite particles within the Cu-Si melt. There is no evidence for any SiC phase formation and the interface is similar to that formed in the B4C/Cu system. The formation of SiC takes place at the B4C/(Cu-15 at% Si) interface (Figure 10).
EDS and WDS quantitative analysis confirm that the interface particles are SiC. It is important to point out that at the first stages of the interaction, when the silicon carbide particles were already formed, the contact an- gle is greater than 90˚. Qualitative line scans (Figure 11(b)) show that a silicon layer covers large portions of the composite SiC-Cu layer. This layer, which lies on the liquid side of the initial interface, formed during the solidification of the molten metal drop and can be attributed to the decreased solubility of Si in molten Cu-Si al- loy with decreasing temperature. At several locations, relatively large SiB3 particles were detected contiguous to the solid, whether Si (Figure 11) or initial B4C, as shown below. These particles apparently nucleated in the course of the melt cooling and provide unambiguous proof of the presence of a significant boron concentration within the melt. The crater that was formed within the boron carbide substrate consists of SiC particles with en- trapped pockets of the Cu-Si-B melt, reminiscent of that of the carbon agglomerates shown in Figure 2(b) and Figure 9. The presence of silicon boride particles, which were formed during solidification and are contiguous to the flat interface (Figure 11(f)), further confirm the enrichment of the melt by boron. The line scan and map- pings underline the complex nature of the molten alloy-boron cabide interface after solidification. Going from the molten Cu-alloy melt towards the substrate, this consists of heterogeneously nucleated silicon boride par- ticles and a quasi-continuous Si layer, both formed during the solidification of the molten alloy due to the great- ly reduced solubility of B and Si in solid Cu. At the initial (prior to crater formation) molten metal boron carbide interface, a quasi-continuous SiC layer is present. The crater volume is filled with additional SiC intermixed with entrapped pockets of solidified Cu-Si-B alloy, with most of the latter adjacent to the roughened boron car- bide substrate surface.
![]()
Figure 7. Sessile drop spreading results at 1423 K in the B4C/ Me = (Cu,Au,Sn)-Si systems.
![]()
Figure 8. The interface of B4C and Sn-8 at% Si melt (after 90 min at 1423 K), a thin continuous SiC layer was formed.
![]()
Figure 9. SEM image at the B4C/(Cu-9 at% Si) interface after 90 min at 1423 K.
![]()
Figure 10. SEM and X-ray mapping images at the B4C/(Cu-15 at% Si) inter- face after 90 min at 1423 K.
![]()
Figure 11. SEM micrograph of the B4C/(Cu-40 at% Si) interface (a). WDS line-scan of boron, carbon, silicon, and copper across the interface (b). Boron, silicon, and copper mappings (c, d, and e, respectively). Silicon boride particles that pre- cipitated in contact with the flat interface near the triple line (f).
3.2.3. Discussion
Alloying the metals with silicon provides another option for the metal-ceramic interaction. Boron carbide exists over a wide range of carbon content, and its interaction with an element with a high affinity to carbon, like Si, leads to the formation of a new carbide phase and to a shift of the boron carbide composition to higher boron content. This interaction depends on the activity of Si in the molten solutions, and on the carbon activity in the boron carbide phase at various compositions. In the binary systems Cu-Si and Au-Si the liquid solutions display a strong negative departure from ideality, whereas the Sn-Si liquid solution has a strong positive departure. Ac- cording to the phase equilibria in the B4C/Me-Si systems (Figure 6), the Me-Si alloys that are in equilibrium with B4C and SiC contain 12 at% Si for the B4C/Cu-Si system and 10 at% Si for the B4C/Au-Si system. On the other hand, even very small Si additions to the liquid Sn (<0.1 at%) lead to SiC formation from the carbon that originated from the boron carbide phase.
For the B4C/(Au-Si) and B4C/(Sn-Si) systems, due to the very limited boron solubility in the melts, boron carbide does not dissociate and a flat interface is observed. A direct interaction between the Si dissolved in the metal and the boron carbide takes place, resulting in the formation of a continuous thin SiC layer that covers the substrate surface. The measured contact angles reflect the characteristic of (Au-Si) and (Sn-Si) melts on silicon carbide. The equilibrium contact angles that were obtained in this study are in a good agreement with the expe- rimental results for the SiC/(Au-Si) and SiC/(Sn-Si) systems reported elsewhere [30] [31] .
The following schema is proposed for the interaction and wetting in the B4C/(Cu-Si) system (Figure 12(d)) and the B4C/(Au, Sn-Si) systems (Figure 12(e)). The first step (Figure 12(a)) consists of the dissolution of bo- ron in the melt, release of carbon, and formation of a crater with graphite agglomerates. At the second stage (Figure 12(b)), silicon dissolved in the melt reacts with graphite and silicon carbide particles are formed. The metal drop does not yet wet the substrate. In the course of the third stage a change of the substrate composition takes place, the boron concentration in the melt increases, boron diffuses to the drop’s periphery and interacts with the substrate surface, and the conditions for wetting are achieved in the vicinity of the triple line (Region A, Figure 12(c), Figure 12(d)). Finally, the metal drop spreads over the sub-stoichiometric boron carbide.
Interface structure and wetting behavior in the B4C/(Me-Si) systems are determined by the solubility of boron in the melt and the interaction between silicon dissolved in the melts with the boron carbide substrate. The equi- librium contact angle in the B4C/(Cu-Si) is affected by the composition of the near-surface layer of the boron carbide that has a higher boron content. Wetting in the B4C/(Au-Si) and B4C/(Sn-Si) systems reflects the forma- tion of a SiC interlayer, and the spreading behavior of the SiC/(Au-Si) and SiC/(Sn-Si) systems.
3.3. The Boron Carbide (Me-Ti, Me=Cu, Au, Sn, Ag) Systems
3.3.1. Thermodynamic Analysis
The sequence of phase formation when pure Ti reacts with stoichiometric boron carbide phase may be analyzed according to the dashed line in the ternary B-C-Ti phase diagram [32] (Figure 13) that links the Ti corner with
![]()
Figure 12. The interaction stages at the boron carbide and Cu-Si melt interface (a-d). (e) The interaction and wetting be- tween boron carbide and (Au, Sn-Si) melt alloys is due to direct wetting of the SiC layer formed at the boron carbide-melt interface.
![]()
Figure 13. Schematic view of the B-C-Ti ternary phase dia- gram at 1400 K. Region I corresponds to TiB2 + graphite + B4C; Region II TiB2 + graphite + TiC, and Region III TiB2 + TiCx.
the point corresponding to boron carbide. Each point on this line defines the relative amounts of the phases that are in equilibrium. At a low Ti/B4C mass ratio, the system is in the three-phase Region (I) TiB2 + graphite + B4C. Thus, TiB2 and graphite are the first phases that are formed at the boron carbide/titanium interface. The forma- tion of the TiC phase takes place only when boron carbide is consumed (or there is no direct contact between melt and substrate) and carbide is in equilibrium with TiB2 and graphite (Region II). At higher Ti content (or if the released free carbon is completely consumed), the conditions for TiB2 coexisting with non-stoichiometric tita- nium carbide phase (TiCx) may be achieved (Region III).
The non-reactive metals do not form any stable carbide or boride phases. Thus, the influence of these metals as solvents for Ti may be attributed to their effect on the respective activities of titanium, carbon, and boron in the liquid solutions. A detailed thermodynamic analysis was done in [28] , which takes into account the condition in which TiC and TiB2 could be formed.
3.3.2. Spreading and Interface Characterization
The values of the equilibrium contact angle for the B4C/(Me-Ti) systems as a function of the Ti content are shown in Figure 14. The addition of small quantities of Ti (less than 1 at%) are needed to achieve wetting of B4C by Cu, Sn, and Ag alloys. For the Au-Ti alloys, wetting conditions were reached when the Ti content was greater than 3 at%.
According to the SEM analysis, the interface structures in the B4C/(Sn-Ti), B4C/(Ag-Ti), and B4C/(Au-Ti) systems were similar. In all these systems, a very thin bi-layered structure was observed between the substrate and the metal drop (for Au-3.4 at% Ti alloy and Ag-0.9 at% Ti alloy, Figure 15(a) & Figure 15(c)). Qualitative WDS scan (Figure 15(b) & Figure 15(d)) shows that the first layer, adjacent to B4C, contains Ti and B, while the second layer contains Ti and C. On approaching the triple line, the thickness of the reaction product layers decreases continuously down to about 0.1 μm, as shown for the B4C/(Ag-Ti) system (Ag-0.9 at% Ti alloy, Fig- ure 16).
The interface between B4C and Cu-Ti melts (Cu-5.4 at% Ti alloy, Figure 17) is quite different from the inter- faces shown above. Due to the strong tendency of B4C to decompose in Cu melts, the interaction at the interface leads to significant substrate dissolution and to crater formation (Figure 17(a)). The crater is filled with graphite agglomerates distributed in the melt, as was previously observed in the B4C/Cu and B4C/(Cu-Si) systems. At some distance from the initial metal/ceramic interface, the presence of a convoluted TiB2 “lace” approximately 30 μm wide was observed (Figure 17(c)). The presence of the discontinuous thin TiB2 layer can be seen just at
![]()
Figure 14. Sessile drop spreading results at 1423K in the B4C/Me = (Ag, Sn, Cu)-Ti systems as a function of the Ti con- tent in the liquid solutions.
![]()
Figure 15. SEM micrographs (a, c) and WDS line scan (b, d) at the B4C/(Me-Ti) interface.
the initial metal/ceramic interface. The titanium content in the crater, between the “lace” and the substrate is practically zero (Cu-1.25 at% Ti alloy, Figure 17(b)), while above the “lace” Ti is still present in the melt (the Ti line scan, compare the Ti level below 20 μm with that beyond 25 μm). The “lace” is detached from the initial ceramic surface along the whole width of the drop, while at the triple line, a TiB2 layer is still connected to the substrate. According to the above results it seems that spreading takes place over the progressively formed TiB2 layer.
3.3.3. Discussion
The interface structure and composition of boron carbide B4C in contact with Me-Ti melts (Me = Sn, Ag, or Au) are determined by the direct interaction between Ti dissolved in the melt and the B4C substrate. Initially, the in- terface is covered by a continuous TiB2 layer and the released free carbon reacts with Ti to form stoichiometric titanium carbide in equilibrium with graphite. Next, after the free carbon is consumed completely, the titanium
![]()
Figure 16. The interface product layer evolution from the middle of the drop to the triple line in the B4C/(Ag-Ti) sys- tem.
![]()
![]()
Figure 17. SEM micrograph, the interface morphology: B4C + graphite + TiB2 + Cu (Ti) Solution are observed (a); WDS line scan of the B4C/(Cu-Ti) interface (b); The location of the TiB2 layer with respect to the drop and the substrate (c).
carbide may change its composition in accordance with the activity of titanium in the melt. At the triple line, the observed spreading angle corresponds to that determined by the contact between TiCx and the Me-Ti melt. The wetting of TiCx by non-reactive metals like Au, Ag, and Sn is affected significantly by the Ti/C ratio (the contact angle decreases with decreasing x value [33] . The detailed thermodynamic calculation was performed in [34] . The calculated titanium carbide compositions (x in TiCx) as a function of the Ti content in the different Me-Ti liquid solutions are shown in Figure 18. At equal titanium concentration, the lowest x value is associated with the Sn-Ti liquid solution and the highest with the Au-Ti melt. These results are consistent with the level of the negative departure the liquid solutions form ideally. The activity of titanium in the Au-Ti melt is significantly lower than in the Sn-Ti and Ag-Ti liquid solutions [35] - [37] . As mentioned, the equilibrium contact angle decreases with decreasing value of x and it is clear that for the same value of the contact angle, the titanium con- centration in liquid Au has to be significantly higher than in liquid Sn and Ag. The results of the thermodynamic analysis are in a good agreement with the experimental results (Figure 14).
Titanium dissolved in liquid Cu may react directly with boron present in the boron carbide at the interface as well as with the dissolved boron in the melt. In both cases the composition of the system is located in the first phase region (I) where TiB2 + graphite + B4C are in equilibrium. Direct reaction of Ti and B4C apparently takes place at the triple line; the metal drop spreads and wetting is achieved thanks to the formation of the TiB2 layer. However, molten Cu continues to dissolve boron from the substrate, free carbon is liberated, and the initial TiB2 layer is detached from the substrate. The absence of Ti below the “lace” (Figure 19) suggests that boron in the melt reacts with dissolved titanium and the new reaction products attach themselves to the already detached tita- nium diboride layer, resulting in the coarsening and convolution of the latter. Since the convoluted and detached TiB2 layer is clearly longer than the length of the substrate to which it was attached, we must conclude that the additional quantity of TiB2 that was formed while the layer was floating in the melt, contributed not only to the thickening but also to the lengthening of the layer. The absence of TiB2 particles, other than the convoluted layer, in the melt suggests that the activation energy for the nucleation of such particles in the melt is high and there is significant surface energy benefit for newly formed TiB2 to contribute to the growth of the existing layer rather than to the nucleation of new particles. It must be mentioned that due to the extremely high affinity of titanium to boron, the formation of TiC doesn’t take place, even though free carbon is present in the vicinity of the boron carbide substrate.
![]()
Figure 18. The composition of titanium carbide that coexists in equilibrium with different Me = (Au, Ag, Sn)-Ti melts at 1423 K.
![]()
Figure 19. Titanium distribution between the TiB2 convoluted “lace” and the B4C substrate, SEM micrograph (a); WDS line scan (b) showing that the Ti concentration between TiB2 and B4C is practically zero.
There is a resemblance between the B4C/Cu Cu-Ti systems, and B4C/Ni, Ni-Ti systems, which were studied by Lin & Sui [4] [5] . Molten Ni dissolves boron carbide but improved wetting isn’t obtained. The addition of Ti reduces the contact angle due to the formation of TiB2 at the substrate/molten drop interface.
3.4. The Boron Carbide/(Me-Al, Me = Cu, Sn) Systems
Among the reactive elements that were added to the non-reactive metals in order to improve wetting, Al, in ad- dition to Si and Ti, was also used in this study. Whereas Si forms stable carbide and Ti forms a stable boride in contact with boron carbide, Al forms a ternary aluminum-boron-carbon phase in contact with boron carbide.
3.4.1. Spreading and Interface Characterization
The equilibrium contact angles as a function of Al content in the melts are presented in Figure 20.
A non-monotonous change of the contact angle in the B4C/(Cu-Al) was observed. SEM images of the inter- faces between B4C and Cu alloys with various Al contents are presented in Figure 21.
A small crater filled with graphite precipitates is observed beneath the solidified drop with 19 at% Al (Figure 21(a)). A similar, but deeper crater was observed at the interface between pure Cu and the boron carbide sub- strate. For Al contents greater than 25 at%, a new phase is formed at the interface (Figure 21(b), Figure 21(c)). The thickness of the interface layer is about 3 - 5 μm. Evidently, this new phase promotes spreading and pre- vents the formation of the crater beneath the liquid drop. According to the WDS analysis, the composition of this phase corresponds to Al8B4C7.
The formation of aluminum borocarbides at the B4C/Al interface was also reported by Lin et al. [38] .
For the alloys containing a high aluminum content (83 at%), the presence of the aluminum boride and alumi- num carbide phases, which have probably been formed during cooling, were detected within the solidified drop (Figure 21(c)). The presence of the ternary Al8B4C7 phase for the Sn-alloys with an Al content greater than 5 at% was also detected in the B4C/(Sn-Al) system. The interface layer in this system is significantly thinner (about 0.5 μm) than in the B4C/(Cu-Al) system.
A non-monotonous change of the contact angle in the B4C/(Cu-Al) system was observed for Al content less than 20 at% (Figure 20). This feature may be related to the formation of the crater at the interface, which is filled by graphite precipitates. The formation of a deep crater at the B4C/Cu interface has been discussed pre- viously and was attributed to the possibility of liquid Cu to dissolve a large quantity of boron, while the solubil- ity of carbon in liquid Cu is extremely low. For the B4C/Cu system the macroscopic contact angle of about 110˚ was observed. This contact angle was considered as the apparent one and its value depends on the crater geome- try. Evidently, aluminum addition to liquid Cu reduces the activity of Cu, the crater becomes shallow, and the apparent contact angle increases. Only when the formation of the interfacial layer takes place (at higher alumi num content) does the contact angle decrease. The differences in the spreading kinetics and in the thicknesses
![]()
Figure 20. The contact angle in the B4C/Cu-Al and B4C/Sn- Al systems at 1423 K as a function of Al content.
![]()
![]()
![]()
Figure 21. The B4C/Cu-Al interface structure. Cu-19 at% Al (a); Cu-40 at% Al (b); Cu-83 at% Al (c). The reaction product at the interface was identified as Al8B4C7. Aluminum carbide and boride inclusions were detected in the solidified drop with high Al contents.
of the interfacial layers for the B4C/(Sn-Al) and B4C/(Cu-Al) systems may be also attributed to the boron solu- bility in the melts. It could be suggested that the interaction at the interface starts from the boron carbide phase decomposition and dissolution of carbon and boron in the melt. This initial stage of the interaction is more promising for the Cu-Al melt because the boron solubility in liquid Cu is significantly higher and the boron transfer from the substrate to the liquid is faster.
3.4.2. Discussion
According to the experimental observations, the wetting behavior in both the B4C/(Sn-Al) and B4C/(Cu-Al) sys- tems is controlled by the formation of the ternary carbide phase at the interface. However, the effect of alumi- num additions for the B4C/(Sn-Al) system is stronger than for the B4C/(Cu-Al) system. The reason for these dif- ferences may be accounted for by thermodynamic properties of the Cu-Al and Sn-Al alloys. It was established that the Cu-Al liquid solution displays a negative departure from ideality, while for the Sn-Al liquid solution this departure is positive [39] [40] . A dominant effect of the thermodynamic properties of the alloys on the wetting behavior is illustrated in Figure 22 where the equilibrium contact angle is plotted as a function of aluminum ac- tivity.
4. Summary and Conclusions
The present report summarizes a comprehensive study concerning the wetting properties of B4C by non-reactive metals (Cu, Au, Ag, Sn). Since these metals do not form stable carbides or borides, they are not able to wet B4C properly. In order to improve wetting three active elements were selected. These elements are able to promote
![]()
Figure 22. The contact angle in the B4C/Cu-Al and B4C/Sn- Al systems as a function of the activity of Al in Cu and Sn melts at 1423 K.
wetting by the formation of new phases at the ceramic/drop interface, according to three different expected reac- tions:
・ The addition of Si, which has relatively high affinity to C, leads to SiC formation and changing the boron carbide composition towards lower carbon content.
・ The addition of Ti, which displays extremely high affinity to B, leads to TiB2 formation and free carbon pre- cipitation at the interface.
・ The addition of Al, which allows forming borocarbide phases at the interface.
In contrast with other investigated non-reactive metals, liquid Cu dissolves 20 - 25 at% of B. When boron was used as alloying addition to Cu, the formation of new phases at the interface was prevented and the effect of bo- ron addition on the wetting behavior was attributed to an altering of the boron carbide composition in contact with boron-containing melts. It was also established that the most important properties of boron carbide that af- fect the wetting phenomena are the relatively low chemical stability and the existence of a wide composition range. The first property determines the possibility of boron carbide to react with liquid metals (by dissolution or formation of new phases) and the second offers an additional degree of freedom to improve its wetting by changing the composition of the ceramic phase.
Acknowledgements
This work was partly supported by the Israel Science Foundation grant 118/03.
NOTES
*Corresponding author.