Stable Oxygen and Deuterium Isotope Techniques to Identify Plant Water Sources ()
1. Introduction
Stable isotope has been developed as a powerful tool for investigating processes in plant-water relations such as recognizing plant water use and responding to different types of water sources, as well as acquiring better understanding of water utilization processes, water use efficiency, pattern, mechanism, and the ability to adopt in arid environments. Plants have to cope with various water sources such as rainwater, soil water, groundwater, sea water and the mixtures of these. Plant water sources are usually characterized by different isotopic signatures (18O/16O and D/H ratios). At present, the measurement of δD, δ18O compositions of various potential water sources and stem water has become a significant means to identify plant water sources [1] .
The analyses of hydrogen and oxygen stable isotope provide an effective approach for studying root water uptake. Zimmermann et al. [2] discovered that there was no hydrogen and oxygen isotope fractionation during root water uptake. Thus, the water absorbed by plant roots can be considered as a mixture of water from different water sources. By comparing the hydrogen and oxygen isotopes in the water from plant xylem and probable water sources, the contributing proportions of different water sources to plants can therefore be confirmed [3] [4] .
There are marked differences in the stable fracture due to various water cycles resulting in the distinct difference of δD and δ18O in various water bodies [5] - [7] . Variation in these sources of water can be as large as 200‰ in a single location [5] , which makes it possible to determine plant water sources and to understand the utilization process of various potential water sources, mechanism, as well as co-existence and competition between the plants and neighbouring plants.
Three potential sources of natural water (precipitation, stream, and groundwater) have been found to vary widely in isotopic composition [8] . Since there is no isotope fractionation associated with the uptake of soil water by roots [1] [9] , the δD and δ18O values of plant xylem water represent a weighted average of all soil water acquired by all functional roots. The soil depth from which xylem water originates can therefore be estimated by comparing the δD or δ18O of plant xylem water with that of the soil water from the different parts of the profile. The palm root system is a system of root fibres, with the roots to a depth of 1 m, but most are at the depth of 15 - 30 cm. Although groundwater surface (water table) is quite deep, active root system is generally located at 5 - 35 cm depth, while the tertiary root is at 10 - 30 cm depth [10] .
2. Material and Method
The research was conducted in Kabun-Aliantan, Tandun (N: 00˚27, 925', E: 100˚49, 219') in Riau, Indonesia. For this purpose, field observation was carried out on the land with the typic Hapludut soil type that has a clay texture, located at 30 - 70 m above the sea level and flat-undulated physiographically. This research was conducted in a plot area of oil palm plantation measuring 50 m × 50 m which consisted of 32 trees. The selection of the trees in the middle of the plot area included as many as 3 trees of oil palm, patterned equilateral triangle as a medium for stem water sampling.
The stem water sampling was conducted on the stem micro sampler type 1906 DVC. The equipment was installed by inserting it into the stem using the first stem pipe end capillary drill which functions to absorb water from the inner wall of palm trunks. The vacuum soil sampler is operated by absorbing water that utilises a suction pipe at the end of the capillary (Figure 1(a)). Meanwhile, groundwater samples were obtained by using a PVC vacuum pipe for one hour with a water machine (Figure 1(b)).
Water sampling was conducted by positioning 5 different depths (20 cm, 50 cm, 100 cm, 150 cm, and 200 cm) around each tree in a circle, with three replications of 1.5 - 2 m from the tree. This water sampling was carried
![]()
![]() (a) (b)
(a) (b)
Figure 1. (a) A micro sampler; (b) PVC vacuum pipe.
out nine times for observation. In addition, water sampling was also conducted for the rainfall, through fall and run-off samples. The water samples were then analysed for the deuterium and oxygen isotopes in each sample observation using a laser spectrometry. The measurement of the isotope ratios of 18O and D Laser Spectroscopy used a device equipped with a microprocessor control analyser with a precision stable isotope ratio analysis. This stable isotope ratio was determined according to the relative difference of a heavier isotope to the lighter isotope (which has greater abundance) as 18O/16O or D/H. From the analysis of the deuterium content (δ2H or δD) and oxygen-18 (δ18O), stable isotopes of water molecules were obtained from information on the processes that had been experienced by the sample water as evaporation or mixing process between two sources of water.
The composition tools included LGR DLT-100 or LWIA (Liquid Water Analyser isotopes), comprising the analysis of the laser system and internal computer, a CTC LC-PAL liquid auto sampler, a small membrane vacuum pump, and an air chamber output channel that passes air through a column of Diorite for removal of moisture. The isotope ratio relative was calculated to that of a standard Mean Ocean Water (SMOW) as astandard Dawson [11] . The deuterium isotope calculation with V-SMOW is as follows:
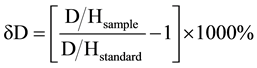
The oxygen isotope calculation with V-SMOW is as follows:
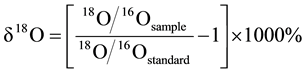
All data were analysed using the statistical LSD test to compare the contents of deuterium and oxygen isotopes in the oil palm trunk and groundwater at each depth. The analysis of oil palm rooting was carried out at the sites to determine the layer that contains the active palm roots which absorb water sources for growth and development. The method of root sampling was done by making the soil profile with a depth of 0 - 200 cm , where the distance sampling was 2, 50 m from the tree trunk, which was then performed on various soil sampling depths (0 - 20 cm , 20 - 50 cm , 50 - 100 cm , 100 - 150 cm , and 150 - 200 cm ) using a ring of soil samples, where the volume of each soil sample was 20 cm × 20 cm × 20 cm . The weighing of soil samples was performed based on the weight of dry samples. This was then carried out based on the criteria for root classifications (primary, secondary, tertiary and quarter) at any depth.
3. Results and Discussion
3.1. Analysis of Deuterium Isotope (δD) of Water Sample
Figure 2 shows that on 17 November 2010, the deuterium (δD) values for rainfall, through fall was close to groundwater at 50 cm depth, with an effective rainfall of 15.90 mm. [8] [11] reported that the isotopic composition of groundwater is a weighted average of a long-term precipitation input. On 6 March 2011, the deuterium (δD) sample for run-off had a value close to the deuterium (δD) sample for stem water, with an effective rainfall of 30.0 mm. On 13 September 2010, the deuterium (δD) value for stem water samples was close to the groundwater sample at 20 cm depth, with effective rainfall of 27.40 mm. On 4 September 2010, the groundwater sample with 100 cm depth was close to the deuterium (δD) for stem water sample, with effective rainfall of 2.60 mm. On 24 October 2010, the deuterium values for stem water samples were close to 150 cm and 200 cm of groundwater depths, with effective rainfall of 14.70 mm. It is important to note that plants can utilize water from precipitation, soil water, run off (including melting), groundwater, fog, and condensate water [5] [12] .
Figure 3 shows that the deuterium concentrations of stem water samples were at the closest point with the deuterium of the groundwater samples at 50 cm depth, followed by the deuterium of groundwater samples at 20 cm depth, the deuterium of groundwater samples at 150 cm depth, the deuterium of ground water samples at 100 cm depth, the deuterium of rainfall samples, the deuterium of through fall samples, the deuterium of ground- water samples at 200 cm death, and the deuterium of run-off samples. The analysis carried out for the hydrogen and oxygen stable isotopes provides an effective approach for studying root water uptake [2] .
In Table 1, the highest concentration of deuterium in the water samples was obtained from the run-off amounting to −58.722‰, where as the lowest concentration of deuterium was presented in the through fall, with the value −52.133‰. Based on the LSD test, it can be seen that the concentration of deuterium (δD) in the stem
![]()
Figure 2. Deuterium isotope on the date of sampling time.
![]()
Figure 3. Deuterium concentrations of groundwater sample.
![]()
Table 1. Deuterium (δD) of water samples.
water samples (−54.633‰) approximated the deuterium concentration of water samples at 20 cm death with −53.878 ‰ and the deuterium concentration of water samples at 50 cm death with −54.644‰. It can be concluded that the value of the deuterium (δD) ratio in all types of treatment in this research did not provide a significantly different effect, whereby the deuterium data sorting concentrations ranged from highest to lowest. The measurement of δD, δ18O composition of various potential water sources and stem water samples was a significant means to identify plant water sources [13] .
3.2. Analysis of Oxygen Isotope (δ18O) of the Water Samples
Figure 4 shows that the water sampling on 25 December 2010 yielded the oxygen isotope (δ18O) values of stem water samples close to the oxygen isotope (δ18O) values of the water samples for rainfall and groundwater sampling at 50 cm depth, with effective rainfall of 45.20 mm. On 17 September 2010, the values of oxygen isotope (δ18O) for the through fall samples were close to the oxygen isotope (δ18O) in the stem water samples, with effective rainfall of 15.90 mm. On 18 February 2011, the oxygen isotope (δ18O) values for the run-off water samples showed that the oxygen isotope (δ18O) values were similar to the oxygen isotope (δ18O) values of the stem water samples in rainy conditions. On 24 October 2010, the samples of groundwater at the depths of 20 cm and 200 cm revealed the oxygen isotope (δ18O) values which were close to the oxygen isotopic (δ18O) values of the stem water samples, with effective rainfall of 14.70 mm. From Figure 5, it can be observed that the samples of groundwater, with the depths of 100 cm and 150 cm, showed the oxygen isotope (δ18O) values that are similar to the oxygen isotope (δ18O) values of the stem water samples, with effective rainfall of 27.40 mm.
Zimmermann et al. [2] discovered no hydrogen and oxygen isotope fractionation during root water uptake; thus, the water was absorbed by plant roots, which can be considered as a mixture of water from different water sources. The results for oxygen isotope (δ18O) concentrations of groundwater sample analysis are displayed in Figure 6.
![]()
Figure 4. Oxygen isotope on the date of sampling time.
![]()
Figure 5. Oxygen isotope concentrations of ground water samples.
![]()
Figure 6. Palm root density in different soil depths.
From Figure 5 above, the concentrations of oxygen isotope of the stem water samples were at the closest point to the groundwater samples at 20 cm depth, followed by the oxygen isotope samples of groundwater at 50 cm depth, the oxygen isotope samples of groundwater at 100 cm depth, the oxygen isotope of throughfall water samples, the oxygen isotope samples of groundwater at 150 cm depth, the oxygen isotope samples of rainfall, the oxygen isotope samples of groundwater at 200 cm depth, and the oxygen isotope samples of run-off. According to Allison [14] , the oxygen of the upper soil water (at 0 - 20 cm depth) was always more enriched due to evaporation than the lower soil horizons and groundwater.
Table 2 reveals that the highest concentration of oxygen isotopes (δ18O) was obtained from the run-off water sample, i.e. −8.067‰. On the other hand, the lowest concentration of oxygen isotopes was found in the water samples at 20 cm depth, with the value −7.171‰. Based on the LSD test, the concentration of oxygen isotopes (δ18O) in the stemwater samples (−7.334‰) approximated the concentration of oxygen isotope at 20 cm depth of the water samples and the concentration of −7.171‰ of the oxygen isotope samples at 50 cm depth, with the value −7.663‰. Therefore, it can be concluded that palm oil plant sabsorb more dominant sources of water at the depths of 0 - 20 cm and 20 - 50 cm for growth and development, respectively. Walker & Richardson [1] stated that by comparing the isotopic composition of water between soil and plant, water uptake can be concluded to have occurred at a depth of 20 to 60 cm, which is in good agreement with the root and soil water potential distributions.
From Table 3 above, the highest mean value in the quarter root and the lowest root in the tertiary can be seen at the depth of 0 - 20. At the depth of 20 - 50 cm, the highest value rates were found in the primary root, where as the lowest values were obtained in the tertiary. At the depth of 50 - 100 cm, the highest value was found in the quarter root. At the depth of 100 - 150, the highest rate was found in the root quarter, while the lowest was found in the primary root. In addition, the average depth of 150 - 200 contained the highest value on the secondary root, where as the lowest value was found in the primary and quarter roots. Based on the information in Table 3 it can be concluded that the rooting of oil palm is more dominant at the depths of 0 - 20 and 20 - 50 cm. According to Fauzi et al. [14] , the roots of secondary, tertiary, and quarter grow parallel to the ground surface roots and even to the tertiary and quarter of the upper layer or to the places that contain lots of nutrients. Furthermore, Iyung [4] stated that most oil palm roots are near the soil surface and only a few roots of palm oil are at the depth of 90 cm. Although ground water surface (watertable) is quite deep, active root system is generally located at the depth of 5 - 35 cm, while tertiary roots are located at the depth of 10 - 30 cm. The result analysis of the root samples at different soil depths in this study is shown in Figure 6.
Figure 6 clearly shows that the rooting of oil palm that is dominated by the quarter root at 0 - 20 cm depth. Hartley [15] stated that the rooting system of palm oil is the root system of fibres, with the roots to a depth of 1 m. However, most roots are found at the depth of 15 - 30 cm. The root system consists of the upper roots of primary, secondary, tertiary, and quarter. The root of the current quarter is the root that absorbs nutrients, water and oxygen.
![]()
Table 2. Oxygen isotopes of water samples.
![]()
Table 3. The root density by soil depth.
4. Conclusion
The analysis of deuterium (δD) and oxygen isotopes (δ18O) in groundwater and water in oil palm trunks provides information on the dynamics of plant utilization of water resources. Based on the Least Significant Difference (LSD) test, no significant value was found in the deuterium and oxygen isotopes in the stem water samples and other samples. However, similar values were obtained at the depths of 0 - 20 cm and 20 - 50 cm in the stem water. It indicates that oil palm absorbs water from the depth of 0 - 50 cm. This result is in accordance with the system of oil palm rooting, i.e. the root quarter (0 - 50 cm) is the most active root of oil palm that absorbs nutrients, water and oxygen.