Effect of Endurance Training on the Autonomic Nervous System Function of Young Male ()
1. Introduction
Autonomic nervous system (ANS) has the function of widely automatic regulation of heart, smooth muscle and endocrine gland. There is an antagonistic effect between sympathethic nerve (SN) and parasympathetic nerve (PN). Dysfunction of ANS plays a pivotal role in cardiovascular disease [1] . Heart rate variability (HRV), resulting from the fine modulation of the sinus node activity by the SN and PN, reflects SN and PN activity of the heart and their balance and coordination [2] . Time and frequency domain analysis of HRV is a harmless method which is most widely used for assessing the autonomic regulation of HR [3] . Proposed by Hines, Brown, et al. [4] in the 1930s, CPT (cold pressor test) is SN provocative test through nervous reflex arc. Cold stimulus activates SN, whose nerve ending releases norepinephrine (NE); on the other hand, it paralyzes PN and hence maximizes epinephrine effect. All these lead to vasoconstriction and elevated BP [5] [6] . The level of BP respond to stress reflects the regulations of ANS. Overly elevated BP (SBP and/or DBP > 20 mmHg) suggests overactive SN, weak autonomic regulation on the cardiovascular system and susceptibility to disease [7] - [9] . Recent researches reveal how HR reacts to stress during CPT also reflects ANS regulation [10] [11] .
In recent years many researches explored how endurance training influenced the ANS, but their conclusions were inconsistent or even contradictory. Most researchers believed that endurance training enhanced PN activity but decreased SN activity, leading to PN dominance [3] [12] - [14] . However, Mandigout [15] , Catai1, et al. [16] believed that endurance training didn’t change ANS activity. Ferdinando, et al. [17] found that 6 months of moderate endurance training and then 3 months of utmost endurance training shifted the initial parasympathetic dominance of the boatmen toward sympathetic dominance. Despite the differences in their methods and subjects, exercise intensity seemed to play an important role in the results. Therefore we inferred that the effect of endurance training on ANS varied with the intensity. However, a systematic study on how the endurance training of different intensities affects ANS has never been reported.
In the present study, young males participated in endurance training of different intensities for 8 weeks. 5 min HRV, CPT, head-up tilt test (HUTT) and NE were used to reveal the effect of moderate, high and utmost intensity endurance training on ANS function.
2. Methods
2.1. Subjects
72 nonsmoking male students, native from sea level, were recruited for the study. All the subjects were healthy as assessed by physical examination. They had the same daily activity and diet, without prior history of professional training. Nobody took any medication during the experiment. The subjects were randomized into moderate intensity (n = 24), high intensity (n = 24) or utmost intensity training (n = 24) group for 8 weeks of endurance training (Table 1). The study protocol was approved by the Ethics Committee of Third Military Medical University, China. Each subject gave written informed consent prior to participation. 8 subjects dropped out from the training because of injury, fear, etc. The final analysis included 23 subjects in the utmost intensity group, 21 in the high intensity group and 20 in the moderate intensity group. There were no significant differences in the characteristics among the three groups.
2.2. Protocol
Utmost intensity group did long distance running according to their predicted maximal heart rate 4 times a week until they were exhausted; moderate intensity group ran 30 min each time, 4 times each week; high intensity group exercised 3 - 5 times a day at a frequency of 4 times per week and each time they ran 1200 m with a 5-min break every 400 m. The Finland Polor Elector™ remote HR monitor recorded their HR when they ran on the 400 m standard runway. The recording of their running HR were used to control the exercise intensity. The formula for calculation was as follows: running HR of utmost intensity group = resting HR + (predicted
![]()
Table 1. Characteristics of study groups at pre and post.
Data are means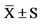 . Pre, pretraining; post, at the end of 8-week training; mid, at the end of 4-week training. Utmost, utmost intensity endurance training; moderate, moderate intensity endurance training; high, high intensity endurance training.
. Pre, pretraining; post, at the end of 8-week training; mid, at the end of 4-week training. Utmost, utmost intensity endurance training; moderate, moderate intensity endurance training; high, high intensity endurance training.
maximal HR – resting HR) × 95% - 100%; running HR of moderate intensity group = resting HR + (predicted maximal HR – resting HR) × 60% - 70%; running HR of high intensity group = resting HR + (predicted maximal HR – resting HR) × 80% - 90%. The whole training process was monitored.
2.3. HRV
Resting 5 min normal RR electrocardiosignal was measured and recorded for time and frequency analysis of HRV before training and at the end of 4 weeks and 8 weeks. The instrument was Model D/SF-I Autonomic Nerve Analyzing Apparatus manufactured by Di Kang Medical Digital Instrument Co. Ltd, Chengdu, China. 24 h before the measurement subjects were free of strenuous exercise, medication, strong tea and coffee. Measurement was taken during a 15-min supine rest in a quiet temperature controlled (15˚C - 20˚C) room.
2.4. CPT
After a 15-min rest, baseline BP was measured at the right radial artery in a sitting position with a mercury sphygmomanometer. In the meantime, baseline HR was measured with an electronic sphygmomanometer (Rijing™, Japan). Then subjects were instructed to immerse their left hands (below the wrist) into a mixture of ice and water (4˚C) for 2 min, with fingers separated. BP and HR were measured again after the 1/2- and 1-minute intervals of immersion of the hand in water at 4˚C [18] .
2.5. HUTT
All subjects were asked to abstain from strenuous exercise for at least 24 h. After a 15-min rest, they lay supinely on a HUTT bed and were connected with electrocardiogram and BP monitor. 5 min later, the head of the bed was raised 70 degrees for 45 min and their HR and BP were recorded every 3 min. This was the basic tilt table test (BTTT). During BTTT, HUTT positive showed the following symptoms or physical signs: apopsychia or precursory symptoms (near unconsciousness, dizziness, blurred vision, nausea, cold sweat, etc.); arterial SBP ≤ 80 mmHg or DBP ≤ 50 mmHg or the average arterial pressure reduction ≥ 25%; the appearance of obvious symptoms caused by sinus bradycardia or boundary cardiac rhythm. The test ended and the bed was put back at the appearance of HUTT positive symptoms. After lying supinely for 10 min, HUTT negative subjects took 0.5 mg Nitroglycerin Tablets and then the head of the bed was raised 70 degrees again. Their HR and BP were recorded every 3 min, this was Nitroglycerin tilt table test (NTTT). The test lasted for 15 min if the subjects didn’t show HUTT positive symptoms [19] . Once HUTT positive symptoms appeared, the bed was put back immediately. Subjects who had serious bradycardia were intravenously injected 1mg Atropine and those with persistent low BP were intravenously injected Atropine and hypertonic glucose.
2.6. Norepinephrine & Epinephrine Detection
Before the training and 5 days after the completion of training, 2 mL venous blood was drawn from ulnar vein of the subjects in the morning when they had empty stomach. The blood was slowly injected into a tube previously anticoagulated with 30 μL 10% EDTA-Na2. After a 15-min centrifugalization at 3000 rpm, the supernate was
drawn and stored in EP tube. 200 μL anticoagulated plasma was put into a 5 mL tube, which was then mixed with 100 μL perchloric acid (0.2 mol/L). After a 20-min centrifugalization at a temperature of 4˚C, 50 μL supernate was drawn from the 5 mL tube and then dropped in the wells. Chromatogram was recorded. NE and EPI concentrations were calculated with specialized software.
2.7. Statistical Methods
Data were analyzed with SSPS (version 13.0) statistical package. After the completion of a normal distribution test, all the data were presented as means ± standard deviation (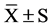 ). Factor analysis was used to compare the physical ability indexes, HRV and CPT at pre, mid and post. As to the blood biochemical indicators at pre and post, t-test was used in intra-group comparison and factor analysis was used in the inter-group comparison. Numeration data analysis was tested by χ2. As a level of significance P < 0.05 was accepted.
). Factor analysis was used to compare the physical ability indexes, HRV and CPT at pre, mid and post. As to the blood biochemical indicators at pre and post, t-test was used in intra-group comparison and factor analysis was used in the inter-group comparison. Numeration data analysis was tested by χ2. As a level of significance P < 0.05 was accepted.
3. Results
3.1. HR
During training, the running HR of the moderate and high intensity group were within the range of P2.5 - P7.5 of resting HR centile. The running HR of the moderate, high and utmost intensity group was 145 - 160 beats/min, 170 - 180 beats/min and >180 beats/min respectively. In the period of training and three months after training, we not observed any training-induced bradycardia.
3.2. Autonomic Nervous System Function
3.2.1
. HRV
Comparison with pre-training. As previously reported, RMSSD, PNN50, HF, SDNN and HFn reflect PN activity; LF and LFn reflects SN activity; LF/HF reflects the balance between SN and PN; HFn and LFn are correction index. In the moderate intensity group a prominent increase in PNN50 and LF (P was 0.022 and 0.011 respectively) and a marked decrease in resting HR (P = 0.00) were noted from pre to mid, but no significant changes occurred to the indexes of other groups. From pre to post, the moderate intensity group showed a significant increase in RMSSD, PNN50, HF, SDNN and LF (P was 0.003, 0.002, 0.002, 0.002, 0.003 respectively), an increasing tendency in HFn but a decreasing tendency in LFn and LF/HF; however, the utmost intensity group demonstrated a significant reduction in HFn (P = 0.012), a marked increase in LF, LFn and LF/HF (P was 0.032, 0.039, 0.015 respectively) and no significant changes in HF, RMSSD, PNN50. The above indexes didn’t change significantly in the high intensity group. Nevertheless resting HR of the three groups decreased significantly from pre to post (P was 0.001, 0.00, 0.009 respectively) (Table 2, Table 3).
Comparison between groups. No significant changes in HRV parameters were found in all groups at pre and mid. But at post, the moderate intensity group showed more significant increases in RMSSD, PNN50, HF, LF and SDNN (P < 0.05 or 0.01) and much greater reduction in LF/HF than the other two groups (P was 0.033, 0.037 respectively). HFn of the moderate intensity group was significantly higher than that of the utmost intensity group (P = 0.012), while the opposite pattern occurred in LFn and LF/HF of the two groups (P was 0.025, 0.015 respectively) (Table 2, Table 3).
3.2.2
. CPT
Compared with pre-training. From pre to mid and from mid to post resting HR of all groups exhibited a decreasing tendency; SBP of the three groups showed no significant changes, but DBP of utmost and high intensity group tended to decrease and that of moderate intensity group markedly decreased (P was 0.001, 0.037 respectively). Compared with pre-training, no significant increases were found in the SBP and SDP of the three groups during CPT; however, while HR of the moderate intensity group slightly increased, that of the utmost intensity group significantly increased (P < 0.05) but HR of the high intensity group markedly decreased (P < 0.05) (Table 4).
Comparison between groups. From pre to post marked differences were not found in SBP and DBP of all groups and their increases. At post HR was much less increased in utmost intensity group during CPT than the other two groups (average P < 0.05) (Table 4).
![]()
Table 2. Time domain indexes of HRV at pre, mid, and post (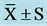 ).
).
Compared with pre-training ▲P < 0.05, ▲▲P < 0.01; Compared with moderate intensity group ★P < 0.05, ★★P < 0.01.
![]()
Table 3. Frequency domain indexes of HRV at pre, mid, and post (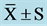 ).
).
Compared with pre-training ▲P < 0.05, ▲▲P < 0.01; compared with moderate intensity group ★P < 0.05, ★★P < 0.01.
![]()
Table 4. CPT at pre, mid, and post (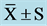 ).
).
▲Compared with pre-training P < 0.05, ★Compared with high intensity group P < 0.05.
3.2.3. HUTT
No positive case in BTTT at pre, but NTTT caused 9 positive cases. At post, BTTT didn’t cause positive case too, NTTT caused 10 positive cases, one of which was quite serious. The subject was better after an emergency treatment. χ2 comparison revealed that no significant inter-group differences were found at pre and post; but HUTT positive cases in the utmost intensity group tended to increase from pre to post though the increase was not statistically significant (Table 5).
3.2.4. Plasma Concentration of NE and EPI
At pre no marked inter-group differences were found in NE and EPI. Plasma NE concentration of the three groups decreased significantly from pre to post (P was 0.016, 0.00, 0.031 respectively); furthermore NE concentration was considerably lower in utmost intensity group than the other two groups (P was 0.001, 0.00 respectively). At post marked inter-group differences were still not found in plasma PEI concentration. There were no significant inter-group differences in the change of NE and EPI from pre to post (Table 6).
4. Discussion
As previously reported, endurance training has an effect on ANS, and ANS function monitoring has been applied to assess training effect and prevent overtraining [13] .
4.1. HRV
Many researches [3] [12] - [14] [17] showed that aerobic endurance training of 8w or more enhanced PN activity but decreased SN activity, resulting in PN dominance. In the present study the moderate intensity group did
Intra-group test was accurately performed with Fisher.
![]()
Table 6. Plasma NE and EPI concentration at pre and post (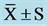 ) (pg/mL).
) (pg/mL).
Compared with pre-training ▲P < 0.05, ▲▲P < 0.01; Compared with utmost intensity group ★P < 0.01.
regular training in accordance with the above mentioned formula. HRV measurement at post also indicated increased PN activity, decreased SN activity and newly established PN dominance. Both LFn and LF are SN activity indexes, but the former decreased whereas the latter increased in the present study. As a marker of both SN and PN activity, the significance of LF remained controversial, but LFn can better represent SN activity [2] . Thus decreased SN activity occurred in the moderate intensity group. On the contrary, Catail et al. [16] did HRV frequency domain analysis and reported that aerobic endurance training didn’t change ANS. However, the sample size in the study was relatively small and more importantly we presume that their subjects mainly did anaerobic endurance training. Because subjects exercised at an intensity exceeding anaerobic threshold were dominated by anaerobic metabolism [20] . In his study though subjects trained with an intensity of 70% - 85% predicted maximal HR, gaseous metabolism detection showed that subjects (age 19 - 29 and age 50 - 59) approximated or exceeded anaerobic threshold when the exercise intensity was 75% predicted maximal HR.
Similar to what Catail found in his so-called “aerobic training”, the high intensity group in the present study did regular training with an intensity of 80% - 90% predicted maximal HR and at post their ANS, SN, PN activity and the interaction between SN and PN remained relatively constant. Mandigou et al. [15] assessed the effect of endurance training at an intensity of 80% predicted maximal HR on children’s ANS by evaluating HRV. They trained for 1 h a day and 3 times a week. 13 weeks later, the absolute value of frequency domain indexes increased in the subjects though marked changes were not found in their LF/HF, LFn, HFn, indicating that SN and PN activity remained relatively constant. This was consistent with our observation that ANS function remained relatively stable when subjects did anaerobic endurance training at an intensity of 80% - 90% maximum HR.
In the endurance training at an intensity of 100% maximum HR, Ferdinando et al. [17] found increased SN activity, decreased PN activity and marked increase in LF/HF. In fact in their study, metabolism of the subjects was anaerobic in nature. Though the subjects of our moderate intensity group also relied on anaerobic metabolism during running, their ANS function changed differently. The two studies had the same training activity, period and age and sex of subjects. Apart from race, the major difference was the intensity of endurance training. HRV measurements by Pichot et al. [13] revealed unchanged PN activity and gradually increased SN activity in subjects receiving overload training (over 100% maximum HR), indicating SN dominance in ANS function. Remarkably similar to the reports of Ferdinando and Picho, we found unchanged (RMSSD, PNN50, HF) or greatly decreased (HFn) PN activity, significantly increased SN activity and newly established SN dominance in utmost intensity group at post. Taking the actual HR and ANS changes of subjects during utmost intensity training into consideration, we concluded that utmost intensity endurance training shifted ANS function toward SN dominance.
4.2. CPT and HUTT
It was reported that BP of African American was over-reacted to stress, which played a role in the etiopathogenisis of hypertension [21] . 8 healthy female African Americans were instructed by Bond et al. [22] to run long distance for 35 min/time, 3 times/week for 6 weeks, at an intensity of 60% - 70% VO2 max. The control group included 5 equivalent sedentary females. CPT revealed decreased SBP and mean arterial blood pressure in the experimental group and no changes in the control group. Therefore they believed that aerobic training could diminish BP reactivity of healthy African Americans to behavioral stress, hence less danger of hypertension. In other words, ANS regulation of subjects was enhanced. In the present study, DBP of all groups decreased at post; BP increase during CPT was not prominent but HR significantly increased in the utmost intensity group, slightly increased in the moderate intensity group and markedly decreased in the high intensity group. Furthermore during CPT HR was much less increased in high intensity group than the other two groups. Utmost endurance training increased HR reactivity to stress, which may lead to greater cardiovascular risk. Consistent with Bond’s research, anaerobic training in the present study enhanced ANS regulation, as indicated by reduced reactivity to stress. However, such reduced reactivity to stress was not found in the moderate intensity group, which was possibly related to the subjects and protocol. Thus further studies are necessary.
Orthostatic intolerance induces vasovagal syncope. Since Kenny et al. [23] introduced HUTT to diagnose syncope of unknown causes in 1986, it has become an effective method for vasovagal syncope diagnosis. Overexcited SN causes reflectively increased PN activity, which in turn induces vasovagal syncope. The mechanism of HUTT is as follows: tilting susceptibles causes central hypovolaemia, poor filling in left ventricle, increased SN activity and over-contracted ventricle, which stimulates mechanoreceptor fiber C in the posterior wall of ventricle; the reflectively excited PN decreases BP and causes bradycardia, blood supply deficiency of brain and attack of syncope [24] . This has been confirmed by HRV measurements of HUTT positive vasovagal syncope patients [25] [26] .
In the present study, both the absolute value of HUTT positive cases and positive rate of utmost intensity group tended to increase, indicating that over-excited SN of these subjects during HUTT induced PN to overreact. This was consistent with altered autonomic balance toward SN dominance in HRV measurements. In addition, no significant changes in HUTT positive cases and the decreasing tendency of HUTT positive rates in moderate intensity group and high intensity group at least suggested that adequate moderate aerobic and anaerobic endurance training had no obvious impairing effect on autonomic regulation.
4.3. Blood Biochemical Indexes
In the present study HRV analysis found decreased resting HR in all groups, marked decrease in DBP of moderate intensity group and no significant change in the BP of utmost and high intensity group at post though SN and PN function of each group changed differently. Obviously neuroregulation mechanism was not sufficient enough to explain this phenomenon.
In 2005 a large-scale Meta analysis consisting of 72 studies, 105 experimental groups and 3926 subjects demonstrated that after aerobic endurance training both resting and ambulatory blood pressure dropped markedly and systemic vascular resistance, plasma NE and PRA (P < 0.05 or 0.01) decreased by 7.1%, 29%, 20%, respectively. These findings suggested that aerobic endurance training lowered BP through various regulatory mechanisms such as SN activity, renin-angiotensin system and reduced vascular resistance, etc. [27] .
In the present study, pronounced inter-group differences were not found in plasma NE and EPI at pre. Plasma NE concentration of moderate intensity group decreased significantly from pre to post, which supported decreased SN activity found in our HRV analysis. It also supported the finding in the above-mentioned Meta analysis that DBP drop was related to ANS function change.
At post NE concentration of utmost intensity group was significantly higher than that of the other two groups, which supported the conclusion from our HRV analysis that compared with moderate and high intensity group SN activity of utmost intensity group was more dominant.
No significant changes in EPI concentration of the three groups, which was inconsistent with the findings in HRV measurements and other blood biochemical indexes in the present study, indicated that training-induced internal environment changes inside our body was rather complex and needed further studies. In a study by Cohn et al. [28] hypertensive patients underwent cycling at 75% VO2 max 6 min/time, 2 times/day for 21 days and significant changes of NE were not found in them but their decreased SN activity was believed to be related to the ameliorated hyperinsulinemia. Takeyama et al. [29] reported that Coronary Artery Bypass Graft patients underwent cycle training at anaerobic level 2 times/day for a year, and plasma NE concentration of both the experimental and control groups decreased since the third week. In addition, Romero et al. [30] found that compared with pre-training resting HR, BP and plasma EPI concentration of toad increased significantly during the training period though NE concentration didn’t show any obvious change and resting EPI/NE ratio raised from 6.3 to 32.9. After the removal of adrenal nerve post-training plasma baseline catecholamine or NE remained unchanged but plasma EPI concentration during exercise was not raised any more, which indicated that at rest EPI was secreted by adrenal gland while NE might be secreted by extra-adrenal chromaffin cells and adrenal gland selectively released the above-mentioned two catecholamines.
In summary, all the methods of assessing the degree of activation of the autonomic system have limitations and they are not interchangeable [1] , in order to avoid the limitation to study the effect of exercise on ANS function, we should use more detection method or index. We noted that after 8 weeks of training plasma NE concentration of all groups decreased significantly, suggesting that endurance training decreased SN activity. However, HRV measurements showed that SN activity of the utmost intensity group increased (LFn, LF/HF). But more importantly, LFn, LF/HF and HFn are only relative values because of calculation principles [2] . In fact, plasma NE concentration in utmost intensity group was still higher than the other two groups though it dropped in all groups. In this sense blood indexes and HRV measurements are concordant with each other. So ANS function changes after endurance training of different intensities reflects the relative change in SN and PN activity.
5. Conclusion
Through the analysis of HRV, CPT, HUTT and blood biochemical indexes, we conclude that the effect of endurance training on ANS function depends on its intensity. Utmost and moderate endurance training results in altered sympathetic and parasympathetic balance towards sympathetic dominance and parasympathetic dominance respectively; whereas high intensity endurance training keeps ANS function relatively stable. How then do we make sense of these phenomena? We need for further research. The newly established dominant position and rebalance of sympathetic and parasympathetic maybe through some possible mechanisms, but the neurohumoral system should be a key component. We will set forth the potential danger or benefit of different intensity endurance training on ANS function in our further study.