1. Introduction
The goal of enzyme technology is to attain the adequacy of stringent industrial requirements to make it better than any other alternative process. This, however, requires scrutinization of many desirable features of the enzyme like, catalytic ability, stability and structural properties. One of the key parameter among such properties is thermal deactivation of enzyme under process condition, which is believed to be a significant factor in many biotechnological processes [1] and in long term processes carried out in a bioreactor. In many cases it will be the consequential factor in deciding on the industrial application of an enzyme. Expeditious inactivation may hinder efficiency of the process in spite of good catalytic ability of the enzyme. Thus, an insight of enzyme deactivation kinetics is essential for better understanding of relation between structure and function of enzymes to enhance the feasibility of biotechnological process [2] . Enzymes are inactivated by many ways by a process where the secondary, tertiary or quaternary structure of a protein changes without breaking covalent bonds or by chemical modification of functional groups of the active site [3] . The enzyme inactivation processes are influenced to a great extent by parameters like temperature, pH, activators and inhibitors. Thus, the study of relationship between enzyme and the process environment is vital in order to accomplish the process manipulation and engineer protein structure. Furthermore, the values of thermodynamic parameters are also helpful in analyzing the stability of proteins.
Cutinase (EC 3.1.1.74) is a cutin hydrolytic enzyme that belongs to the family serine hydrolases [4] . Apart from its natural substrate cutin, they can act on multifarious substrates such as esters, long and short chain fatty acids, triglycerides, biopolymers and share some vital catalytic properties of lipase and esterase. Because of its unique catalytic nature, it is being considered as one of the industrially important enzymes. Some of the useful applications of cutinase include hydrolysis of fats and oils, degradation of synthetic polymers like polyethylene terephthalate [5] [6] , biodegradation and detoxification of fatty acid based toxins [7] , esterification and transesterification reactions [8] . It has a potential to be an efficient biocatalyst in the food industry for synthesis of flavors, petrochemical industry for synthesis of biodiesel and preparation of house-hold detergents [9] . Both fungal and bacterial species have been reported to produce cutinase [4] [10] -[15] .
Thermobifida fusca is an aerobic, moderately thermophilic, filamentous soil bacterium that is a major degrader of plant cell walls in heated organic materials [16] . Recently, a few studies describing production, biochemical characterization and application of cutinase from T. fusca in various fields such as cotton scoring, degradation of polymeric substrates have been published [5] [6] [12] [17] .
In our previous studies on biochemical properties of two homologous cutinases, Cut1 and Cut2 from T. fusca NRRL B-8184, we observed that both the cutinases are thermostable, active in a broad range of pH and highly resistant to many surfactant and organic solvents, which could have great biotechnological promise in many industrial applications [12] . Furthermore, our studies on biochemical and structural properties of these two homologous enzymes also revealed that despite of 93% identity among them at amino acid level, they showed different substrate specificity, biochemical and biophysical properties [12] [18] . In addition, fluorescence and CD spectral analysis of the equilibrium unfolding behavior in presence denaturants also revealed that Cut2 is structurally more stable in comparison to Cut1 and the hydrophobicity and surface electrostatic properties of these two enzymes are different [18] . However, no information is available on thermodynamic properties of these enzymes, which indicates the importance of present thermodynamic study for these two enzymes.
Thus, the present work focuses on study of combined effect of pH and temperature on purified, recombinant cutinases from Thermobifida fusca NRRL B-8184. It also deals with deactivation kinetics and thermodynamic parameters (∆H*, ∆S*, ∆G* and activation energy) of thermal deactivation.
2. Materials and Methods
2.1. Chemicals
All the chemicals used in this study were of analytical grade purchased from Sigma Aldrich Co. India. T. fusca cutinase, Cut1 and Cut2 used in the present study was previously cloned into pET22b(+), expressed in E. coli BL21 (DE3), purified to homogeneity and characterized in our laboratory [12] .
2.2. Cutinase Assay
Cutinase activity against p-nitrophenyl butyrate (pNPB) was determined according to the method described earlier [12] .
2.3. Thermal Deactivation Study
In order to study the thermal stability of cutinase in different pH, the enzyme was incubated at four different temperatures in the range of 45˚C and 80˚C. The pH of buffer containing the purified enzyme was adjusted to four different levels, viz., 6.0, 7.0, 8.0 and 9.0. The enzyme samples were deactivated at various combinations of pH and temperature (Table 1). Aliquots of samples were collected at different intervals of time and were assayed for the residual enzyme activity by pNPB assay. All the experiments were performed in duplicates until and otherwise mentioned.
2.3.1. Estimation of Deactivation Rate Constant
The following first order expression was used to account for the zero activity at a particular temperature and at specified incubation time.
 (1)
(1)
where, kd is enzyme deactivation rate constant (h−1); t is incubation time (h); Et is the enzyme activity (U/ml) at time t and E0 is initial enzyme activity (U/ml) at time t = 0, The values of kd were calculated from the plot of ln(Et/E0) vs. t at a particular temperature.
The half-life of an enzyme was defined as the time required by the enzyme to lose half of its initial activity and can be expressed by the following equation.
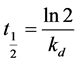 (2)
(2)
2.3.2. Estimation of Thermodynamic Parameters for Cutinase Deactivation
In order to obtain the change in enthalpies (∆H*) and change in entropies (∆S*) during enzyme deactivation
![]()
Table 1. Effect of temperature at different pH on deactivation constant (kd) and half life time (t1/2) of Cut1 and Cut2.
Results are the average of two experiments with ± SD.
process, it is necessary to make use of the theory of absolute reaction rates [19] [20] . The central point of this theory is that the rate of any reaction at a given temperature depends only on the concentration of an energy-rich activated complex, which is in equilibrium with the inactivated reactants. The deactivation constant is expressed by the following equation.
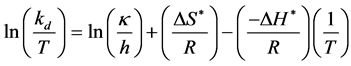 (3)
(3)
where, kd is enzyme deactivation rate constant (h−1); κ is Boltzmann constant (1.38 × 10−23 J/K); h is Plank’s constant (6.626 × 10−34 J×s) ΔH* is change in enthalpy (J/mol); ΔS* is change in entropy (J/mol/K); R is gas constant (8.314 J/M/K) and T is temperature (K). The values of ΔH* and ΔS* were calculated from the slope and intercept of the plot of ln(kd/T) versus 1/T, respectively. Values of change in free energy (ΔG*) were further estimated by the following relationship.
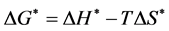 (4)
(4)
where, ΔG* is change in free energy (J/mol).
The activation energy (EA) was calculated from the Arrhenius equation as:
 (5)
(5)
where, EA activation energy (J/mol) and k0 is frequency factor (h−1).
The values of EA and k0 were estimated from the slope and intercept of the plot of ln(kd) versus 1/T, respectively.
3. Results and Discussion
3.1. Thermal Deactivation at Different pH and Temperature
Cut1 and Cut2 were deactivated under various combinations of pH and temperature as discussed in “Materials and Methods”. The extent of deactivation was measured by the deactivation rate. The deactivation rate is proportional to the active enzyme concentration (expressed in terms of specific activity), and kd (deactivation rate constant) is the proportional constant. The deactivation process was modeled as first-order kinetics and the deactivation rate constant was evaluated. The effect of temperature on half-life time has been studied and the results are shown in Table 1 for Cut1 and Cut2. The minimum value of kd observed for Cut1 and Cut2 are 0.045/min and 0.039/min, respectively. The combinations of pH and temperature at which the above mentioned minimum deactivation rate constant have been observed are 8˚C and 45˚C for both Cut1 and Cut2. The deactivation process was found to be faster at pH below 7 and above 8 for both the cutinases. However, the rate of deactivation was faster for Cut1 in comparison to Cut2 (Table 1). According to the 2D electrophoresis, the pI of the Cut1 and Cut2 was determined to be 7.5 and 7.7, respectively (data not shown) thus, maximum stability was achieved close to the pI of the enzyme [21] . Similar observations have been made for cutinase from F. solani and other enzymes [21] [22] . At pH values above and below pI, the net charge may lead to an electrostatic destabilization of the protein, hence the lower thermal stability.
It was also observed that with increase in the temperature above 70˚C, the deactivation occurs faster, irrespective of pH for both the cutinases. Furthermore, the observation of interrelationship between conformational stability and enzyme activity suggested that in naturally occurring enzymes one cannot expect to find stability at temperatures far above than that of growth of an organism [23] . The results obtained in the present study also indicate that optimum pH and temperature lie near that of the growth condition.
It was found that maximum half-life time of Cut2 was 17.59 h, showing that this enzyme is more stable than Cut1 (15.3 h) at optimum conditions of pH and temperature (pH 8 and 45˚C, respectively).
3.2. Enthalpy and Activation Energy Change during Deactivation in Varying pH
In order to understand the behavior of enzyme molecules in different physiological conditions change in enthalpy (∆H*) and activation energy (EA) was investigated. In general, thermal denaturation of enzymes occurs in two steps i.e., where N is native enzyme, U is unfolded inactive enzyme which could be reversibly refolded upon cooling and I is inactivated enzyme formed after continuous exposure to heat for long time, which cannot be recover upon cooling [24] [25] . Enthalpy change and deactivation energy of Cut1 and Cut2 were calculated within a temperature range of 45˚C to 80˚C. It has been reported that enthalpy change of enzymes should be in the range of 20 to 150 kJ∙mol−1 [26] . As depicted in Table 2, ∆H* of Cut1 and Cut2 deactivation was within this value in the range of buffers studied at all the temperatures, which indicates that these enzymes maintained their rate of reaction even during incubation at different pH and temperatures. Furthermore, enthalpy of Cut1 and Cut2 increased as pH increased (Table 2) till pH 8. However, there was a decrease in enthalpy of Cut1 and Cut2 at pH 9. This result shows that both the cutinases were more stable at pH 7 and 8. However, it was interesting to observe that ∆H* value of Cut2 was ~1.5 fold higher than Cut1 in the range of pH studied, which indicates that Cut2 is thermodynamically more stable than Cut1. Enthalpy derives from the energy of the non-covalent interactions within the polypeptide chain, the hydrophobic interactions, H-bonds and ionic bonds. Thus, the substantial difference in the enthalpy for Cut1 and Cut2 indicates that there is a considerable tertiary structural difference between Cut1 and Cut2. Similar structural stability difference was observed for these enzymes with guanidine hydrochloride based equilibrium unfolding studies [18] .
The temperature dependency of first-order deactivation rate constant was studied by Arrhenius equation (Eq. 8). The activation energy (EA) estimated are shown in Table 2. It was observed that the deactivation energy is maximum at optimum pH for both Cut1 and Cut2, however, Cut2 showed ~1.7 fold higher deactivation energy at pH 6 and 7 and ~1.4 fold higher deactivation energy at pH 8 and 9 in comparison to Cut1 (Table 2). The higher deactivation energy suggests that Cut2 require more energy to get deactivated compare to Cut1. The differential thermodynamic properties of the two cutinases may be linked with surface electrostatic properties of the two enzymes and the dissimilarity in the N-terminal amino acid regions [18] . It has also been observed for the F. solani cutinase that N-terminal region has a crucial role in the unfolding of the cutinase [21] [27] .
It has also been reported that unfavorable charge distribution on the surface of proteins is capable of destabilizing the protein [28] . Furthermore, the surface electrostatic interactions are believed to contribute positively to the protein stabilization by forming ionic interactions (salt bridges) between residues carrying opposite charges [21] . It has been observed that surface electrostatic properties of the two cutinases of T. fusca are fairly different and due to such differential properties these enzyme possess different stability and unfolding behavior in detergent, GdnHCl [18] . Thus, the difference in the stability of the two homologous proteins might be due to differential distribution of noncovalent electrostatic and hydrophobic interaction energies in Cut1 and Cut2.
![]()
Table 2. Estimated thermodynamic parameters during thermal deactivation for Cut1 and Cut2.
#The temperature range is 45˚C - 80˚C, Results are the average of two experiments with ± SD.
3.3. Entropy and Free Energy Change during Deactivation in Varying pH
The measurement of entropy change during unfolding of protein molecule is very much helpful in enhancing the thermostability of proteins of known 3-dimensional structure. This can be achieved by selective amino acid substitutions, which decreases the configurational entropy change of unfolding thereby increases the stability of the protein molecule [29] . In the present study, it was observed that the entropy values are negative for both the enzymes in all the cases (Table 2), which is unique in biocatalytic systems. The possible reason for negative entropy could be due to the formation of charged particles around the enzyme molecule and the ordering of solvent molecules or compaction of the enzyme molecules [25] [30] . Both Cut1 and Cut2 showed increase in entropy with increase in pH (Table 2). The probable reason is that enzyme gets unfolded during deactivation with the increase in pH or it may be due to the ordering of solvent molecules. Though the difference in the entropy change is marginal in the range of pH studied, apparently, Cut1 had lower entropy in comparison to Cut2. Furthermore, the negative ∆S* also signifies that the transition states of the cutinases were found to be ordered. Similar results have been observed for the amylase of Bacillus lichiniformis [31] , the chitinase of T. harzianum [10] and the chitinase from Pantoea dispersa [25] . This result also indicates that cutinases were altered in the direction of partially unfolded transition state but the flexibility implies decreased conformational entropy of the folded state which is favorable to thermodynamic stability. Thus, it may be concluded on the basis of entropy that the mechanism of deactivation for Cut1 and Cut2 is more or less similar although the stability of Cut1 and Cut2 are different, with Cut2 to be thermodynamically more stable than Cut1.
Voordouw et al. (1976) [32] proposed that kinetic thermal stability should be used for defining thermostable enzymes. In addition, these authors emphasize that resistance of enzymes to thermal denaturation is due to the ‘intrinsic’ contribution of the polypeptide chain (i.e. hydrophobic interactions, hydrogen bonding and ionic stabilization). For both the cutinases, ∆G* increased with increasing temperature (Table 2). However, there was a marginal difference in ∆G* and both the cutinases showed almost similar free energy change with increasing temperature. Moreover, there was no or very small difference in the ∆G* value in the range of pH studied implies that pH had negligible effect on denaturing the enzymes or in other words; both the cutinases are active in broad pH.
4. Conclusion
In the present study, the pH and temperature dependent thermal deactivation and thermodynamic properties of the cutinases from T. fusca have been elucidated to gain a new insight into the structure and functional relationship of the enzyme in view of its stability toward pH and temperature. The present study also demonstrated that two highly homologous cutinases show different thermodynamic behavior. Further, detailed structural and biophysical studies with differential scanning calorimetry and high resolution NMR and solving the crystal structure and biophysical analysis might shed light on the exact reason for these differences.
Acknowledgements
Authors acknowledge a financial support from DST through project for carrying out the experiments.
NOTES
*Corresponding author.