Synthesis and Characterization of New Chiral Monoanionic [ON] Ancillary Phenolate Ligands ()
1. Introduction
Ligand design has been of great importance in asymmetric catalysis and ring opening polymerization of lactones due to the fact that the reactivity and selectivity of metal catalysts are largely determined by the ancillary ligands [1] -[7] . One of the challenges is to derive efficient chiral catalysts for asymmetric induction in different substrates with subtle variations. Since it is not expected that a single catalyst will work for a wide range of substrates, an efficient strategy towards new catalysts would be the design of a search pathway that provides access to a large number of structurally similar ligands with tunable yet diverse substituents [8] . Indeed, many researchers have sought to develop asymmetric catalysts by screening a large pool of chiral ligands [9] -[12] .
Aminophenolate ligands have received great attention in metal-catalyzed ring-opening polymerization (ROP) of lactones due to the potential to fine tune the steric and electronic properties by varying the substituent groups and pendant side-arms, as well as their inexpensive synthetic strategies [13] -[22] . In fact, well-defined metal complexes of phenolate as ancillary ligands have been studied intensively to investigate the electronic and steric properties of the central metal and their effects in ROP of cyclic esters [13] -[46] . However, given their widespread application, it is somewhat surprising that the chiral variants of aminophenolate ligands are relatively lacking in the literature. The introduction of an aromatic ring in the pendant side arm, the resonance in the backbone is attenuated in comparison with the regular ligands and this may offer some unique opportunities for electronic differentiation and stereocontrol upon coordination [47] . Of particular interest are ligands with chiral pendant substituents that are in close proximity with the open coordination site at the periphery where catalysis occurs. Discussed herein is the synthesis of new chiral [ON] aminophenolate ligands as potential ancillary ligands in asymmetric catalysis and ring-opening polymerization of lactones.
2. Experimental
2.1. General
Deuterated solvents were purchased from Cambridge Isotope Laboratory and used as received. 2,4-di-tert- butylphenol, 2,4-dimethylphenol, 2,4-di-tert-pentylphenol, 37 wt% formaldehyde, and N-methylbenzylamine were purchased from Acros Organic and used as received. 2-tert-butyl-4-methylphenol and (+)-bis-[(R)-1-phe- nylethyl] amine were purchased from Aldrich while 4-tert-butyl-2-methylphenol was purchased from Fluka and used as received. All 1H and 13C NMR spectra were recorded on a JEOL ECX-300 MHz NMR spectrometer and referenced to CDCl3. Elemental analyses were performed by Midwest Microlab, Indianapolis, IN. Melting points were obtained on a Mel-Temp apparatus and are uncorrected.
2.2. Synthesis of Ligands
2.2.1. HLa
2,4-Di-tert-butylphenol (1.834 g, 8.87 mmol), 37 wt% formaldehyde (0.266 g, 8.87 mmol), and (+)-bis-[(R)-1- phenylethyl] amine (2.000 g, 8.87 mmol) were dissolved in ethanol (13 mL). The resulting solution was heated at reflux for 18 h and then cooled to room temperature. Solvent and water were removed using high vacuum Schlenk line to obtain pale yellow oily compound, which was purified by column chromatography (5% ethyl acetate and 95% hexane). (2.198 g, 55.9%). Elemental analysis: (Found: C 83.22, H 9.03, N 3.38. C31H41NO requires C 83.922, H 9.315, N 3.157. 1H NMR (300 MHz; CDCl3; 298 K) 1.30 (s, 9H, ArtBu), 1.37 (d, 3H, J = 6.87 Hz, ArCH(Me)N), 1.44 (s, 9H, ArtBu), 1.52 (d, 3H, J = 6.87 Hz, ArCH(Me)N), 3.71 (d, 1H, J = 14.76 Hz, ArCH2N), 4.12 (q, 1H, J = 6.87 Hz, ArCH(Me)N), 4.29 (d, 1H, J = 14.76 Hz, ArCH2N), 4.84 (br, 1H, ArCH(Me)N), 6.64 (d, 1H, J = 8.22 Hz, ArH), 7.12 (d, 1H, J = 8.22 Hz, ArH), 7.30 - 7.40 (br, 10H, ArH), 11.12 (s, 1H, ArOH). 13C{H} NMR (75 MHz; CDCl3; 298 K) 24.9 (ArCH(Me)N), 29.7 (ArCMe3), 31.8 (ArCMe3), 34.7 (ArCMe3), 34.9 (ArCMe3), 51.3 (ArCH(Me)N), 53.3 (ArCH2N), 116.0, 123.6, 126.9, 127.1, 128.3, 128.5, 128.6, 142.9, 152.1 (all ArC).
2.2.2. HLb
2-Tert-butyl-4-methylphenol (2.189 g, 13.31 mmol), 37 wt% formaldehyde (0.400 g, 13.31 mmol), and (+)-bis- [(R)-1-phenylethyl] amine (3.000 g, 13.31 mmol) were dissolved in ethanol (13 mL). The resulting solution was heated at reflux for 18 h and then cooled to room temperature. Solvent and water were removed using high vacuum Schlenk line to obtain pale yellow oily compound, which was dried at 70˚C.(2.872 g, 80.6%). Elemental analysis: (Found: C 83.63, H 8.77, N 3.42. C28H35NO requires C 83.74, H 8.785, N 3.49. 1H NMR (300 MHz; CDCl3; 298 K) 1.34 (d, 6H, J = 6.87 Hz, ArCH(Me)N), 1.47 (s, 9H, ArtBu) 2.32 (s, 3H, ArMe), 3.58 (q, 2H, J = 6.87 Hz, ArCH(Me)N), 4.09 (br, 2H, ArCH(Me)N), 6.62 (d, 1H, J = 7.92 Hz, ArH), 6.90 (d, 1H, J = 7.92 Hz, ArH), 7.26 - 7.38 (br, 10H, ArH), 11.05 (s, 1H, ArOH). 13C{H} NMR (75 MHz; CDCl3; 298 K) 16.7 (ArCMe3), 24.9 (ArCMe3), 31.9 (ArCH(Me)N), 34.2 (ArCH(Me)N), 55.4 (ArCH2N), 114.9, 123.8, 124.1, 127.1, 127.3, 128.3, 128.8, 143.1, 145.2, 152.2 (all ArC).
2.2.3. HLc
4-Tert-butyl-2-methylphenol (1.510 g, 8.87 mmol), 37 wt% formaldehyde (0.266 g, 8.87 mmol), and (+)-bis- [(R)-1-phenylethyl]amine (2.000 g, 8.87 mmol) were dissolved in ethanol (13 mL). The resulting solution was heated at reflux for 18 h and then cooled to room temperature. Solvent and water were removed using high vacuum Schlenk line to obtain pale yellow oily compound, which was dried at 70˚C.(2.133 g, 59.9%). Elemental analysis: (Found: C 83.47, H 8.95, N 3.68. C28H35NO requires C 83.74, H 8.785, N 3.49. 1H NMR (300 MHz; CDCl3; 298 K) 1.41-1.46 (s, 15H, ArtBu, ArCH(Me)N), 2.44 (s, 3H, ArMe), 3.67 (q, 2H, J = 6.87 Hz, ArCH(Me)N), 4.36 (br, 2H, ArCH(Me)N), 6.84 (d, 1H, J = 8.25 Hz, ArH), 7.20 (d, 1H, J = 8.25 Hz, ArH), 7.33 - 0.46 (br, 10H, ArH), 10.52 (s, 1H, ArOH). 13C{H} NMR (75 MHz; CDCl3; 298 K) 16.7 (ArCMe3), 24.9 (ArCMe3), 31.9 (ArCH(Me)N), 34.2 (ArCH(Me)N), 55.4 (ArCH2N), 114.9, 123.8, 124.1, 127.1, 127.3, 128.3, 128.8, 143.1, 145.2, 152.2 (all ArC).
2.2.4. HLd
2,4-Di-tert-butylphenol (3.522 g, 17.069 mmol), 37 wt% formaldehyde (0.512 g, 17.069 mmol), and N-methyl- benzylamine (2.068 g, 17.069 mmol) were dissolved in ethanol (30 mL). The resulting solution was heated at reflux for 18 h and then cooled to room temperature. Crystallization from the saturated ethanol solution at room temperature yielded white solid, which was dried under high vacuum at 70˚C (4.510 g, 77.8%). Mp: 132.6˚C - 132.9˚C. Elemental analysis: (Found: C 80.97, H 9.67, N 4.15. C23H33NO requires C 81.37, H 9.80, N 4.13%). 1H NMR (500 MHz; CDCl3; 298 K) 1.43 (s, 9H, ArtBu), 1.64 (s, 9H, ArtBu), 2.29 (s, 3H, ArCH2NMe), 3.61 (br, 2H, ArCH2NMe), 3.78 (br, 2H, ArCH2NMe), 6.92 (s, 1H, ArH), 7.29?7.40 (br, 6H, ArH), 11.17 (br, 1H, ArOH).13C{H} NMR (125 MHz; CDCl3; 298 K) 29.7 (ArCMe3), 31.4 (ArCMe3), 34.2 (ArCMe3), 34.4 (ArCMe3), 41.2 (ArCH2NMe), 60.9 (ArCH2NMe), 62.1 (ArCH2NMe), 121.4, 122.9, 123.4, 1275, 128.5, 129.5, 135.7, 137.3, 140.6, 154.3, 176.6 (all ArC).
2.2.5. HLe
2,4-Di-tert-pentylphenol (2.000 g, 8.535 mmol), 37 wt% formaldehyde (0.256 g, 8.535 mmol), and N-methyl- benzylamine (1.034 g, 8.535 mmol) were dissolved in ethanol (30 mL). The resulting solution was heated at reflux for 18 h and then cooled to room temperature. Crystallization from the saturated ethanol solution at ?10˚C (freezer) yielded white solid, which was dried under high vacuum at 50˚C (2.490 g, 79.4%). Mp: 73.9˚C - 74.1˚C. Elemental analysis: (Found: C 81.61, H 9.99, N 3.90. C25H37NO requires C 81.69, H 10.15, N 3.81%). 1H NMR (300 MHz; CDCl3; 298 K) 1H NMR (500 MHz; CDCl3; 298 K) 0.74 (t, 3H x 2, J = 7.50 Hz, ArCMe2CH2Me), 1.31 (s, 6H, ArCMe2CH2Me), 1.47 (s, 6H, ArCMe2CH2Me), 1.65 (q, 2H, J = 7.50 Hz, ArCMe2CH2Me), 2.03 (q, 2H, J = 7.50 Hz, ArCMe2CH2Me), 2.27 (s, 3H, ArCH2NMe), 3.57 (br, 2H, ArCH2NMe), 3.79 (br, 2H, ArCH2NMe), 6.86 (s, 1H, ArH), 7.16 (s, 1H, ArH), 7.31 - 7.41 (br, 5H, ArH), 11.04 (br, 1H, ArOH). 13C{H} NMR (125 MHz; CDCl3; 298 K) 9.2 (ArCMe2CH2Me), 9.6 (ArCMe2CH2Me), 27.7 (ArCMe2CH2Me), 28.6 (ArCMe2CH2Me), 33.0 (ArCMe2CH2Me), 37.3 (ArCMe2CH2Me), 38.5 (ArCMe2CH2Me), 41.1 (ArCH2NMe), 60.8 (ArCH2NMe), 62.1(ArCH2NMe), 121.1, 124.1, 125.1, 127.5, 128.4, 129.5, 133.9, 137.6, 138.7 154.0 (all ArC).
2.2.6. HLf
2,4-Di-methylphenol (10.421 g, 85.3 mmol), 37 wt% formaldehyde (2.56 g, 85.3 mmol), and N-methylbenzy- lamine (10.342 g, 85.3 mmol) were dissolved in methanol (20 mL). The resulting solution was heated at reflux for 18 h and then cooled to room temperature. Solvent and water were removed using high vacuum Schlenk line to obtain pale yellow oily solid. Recrystallization from ethanol at ?10˚C (freezer) yielded off white solid, which was dried under high vacuum at room temperature. (20.45 g, 96.5%).Mp: 33.5˚C - 33.6˚C. Elemental analysis: (Found: C 80.01, H 8.13, N 5.50. C17H21NO requires C 79.960, H 8.289, N 5.485. 1H NMR (300 MHz; CDCl3; 298 K) 2.22 (s, 3H, ArMe), 2.23 (s, 3H, ArMe), 2.24 (s, 3H, ArCH2NMe), 3.61 (s, 2H, ArCH2NMe), 3.71 (s, 2H, ArCH2NMe), 6.67 (s, 1H, ArH), 6.88 (s, 1H, ArH), 7.29 - 7.37 (br, 5H, ArH), 10.97 (br, 1H, ArOH). 13C{H} NMR (125 MHz; CDCl3; 298 K) 15.8 (ArMe), 20.6 (ArMe), 41.2 (ArCH2NMe), 61.1 (ArCH2NMe), 61.7 (ArCH2NMe), 120.9, 124.8, 126.8, 127.7, 128.9, 129.5, 130.7, 137.0, 153.6 (all ArC).
3. Result and Discussion
The chiral ligands HLa, HLb and HLc (Figure 1) were synthesized via Mannich condensation reactions using inex- pensive substituted phenols, formaldehyde and (+)-bis-[(R)-1-phenylethyl] amine in refluxing ethanol (Scheme 1).
![]()
Figure 1. New multidentate ancillary phenolate ligands.
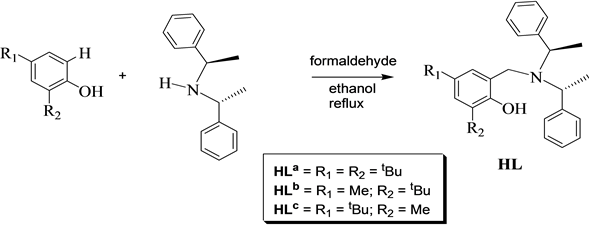
Scheme 1. Synthesis of chiral phenolate ligands via Mannich condensation reactions.
These compounds were purified by column chromatography to obtain oily products in moderate to high yields. The corresponding products were then characterized using NMR and elemental analysis to ascertain the structures. The use of different phenolic substituents (methy, butyl and pentyl) will provide a library of compounds sui- table for metal catalytic investigations in organic functional group transformations and polymerization reactions.
Meanwhile, the non-chiral ligands HLd, HLe and HLf (Figure 1) were synthesized in a similar manner using substituted phenols, formaldehyde and N-methylbenzylamine in refluxing ethanol. These compounds were purified by recrystallization to obtain white solids in high yields. The solids were also characterized using NMR and elemental analysis. Yields of the non-chiral ligands were generally higher (77% - 96%), presumably due to the use of a less bulky N-methylbenzylamine compared to the bulky (+)-bis-[(R)-1-phenylethyl] amine and also due to easier purification methods.
Deprotonation of the ligands and attachment to zinc, tin and palladium metals would offer new research opportunities in asymmetric synthesis and metal catalyzed ring-opening polymerization of lactones. There is great interest in investigating the effect of one stereogenic center in conjunction with phenolic bulky substituent on catalytic selectivity. These ligands are expected to be bidentate with the possibility of having a tridentate coordination via the phenyl pendant arms.
4. Conclusion
New chiral monoanionic [ON] ancillary phenolate ligands with varying pendant arms have been synthesized and characterized via nuclear magnetic resonance spectroscopy (1H and 13C) and elemental analysis. The synthesized ligands are suitable candidates for applications in asymmetric catalysis and ring-opening polymerization of lactones.
Acknowledgements
The authors are grateful to Duke Energy Advance SC for research funding.
NOTES
*Corresponding author.