Highly Efficient, One Pot Synthesis and Oxidation of Hantsch 1,4-Dihydropyridines Mediated by Iodobenzene Diacetate (III) Using Conventional Heating, Ultrasonic and Microwave Irradiation ()
1. Introduction
Development of highly efficient synthetic methodologies for the construction of biologically important compounds is one of the challenges to medicinal and organic chemist. One of the most relevant approaches to synthetic efficiency is based on multi component reactions (MCRs) which combine three or more substrates, either simultaneously, leading to domino processes [1] , or through the sequential reactions without isolating intermediate species. MCRs offer several advantages such as atom economy, minimized waste generation, because of the reduction in the number of work-up, extraction and purification stages.
Use of ultrasonic irradiation and microwave irradiation as alternative sources of energy has proved to be one of the stepping stone towards the green syntheses as it offers advantage of enhanced reactivity, shorter reaction times and higher yields of pure products compared to the traditional heating methods [2] [3] . The efficiency and expediency of MCRs leading to heterocyclic scaffolds can be increased to several times using one pot reaction profiles, greener catalysts, ultrasonic, and microwave irradiation.
1,4-Dihydropyridines (1,4-DHPs) belong to a class of nitrogen containing heterocycles having a six-mem- bered ring. Much attention has been devoted to explore their pharmacological activities. A considerable portion of today’s efforts in dihydropyridine chemistry is expanded in synthesizing reduced form of nicotinamide ade- nine dinucleotide (NADH) mimics, exploring the reactions and mechanisms of these compounds, and utilizing them in a variety of synthetic reactions. Newly synthesized substituted 1,4-DHPs possess other pharmacological activities such as antitumor [4] , bronchodilating [5] , antidiabetic [6] , neurotropic [7] , antianginal [8] and P-glyco protein Inhibitors [9] . The benign environmental character and easy commercial availability makes hypervalent iodine (III) reagents increasingly important for the oxidation of organic molecules [10] -[18] . These days much work has been done to explore the oxidation ability, their electrophilic properties and to develop novel reaction using hypervalent iodine compounds [19] .
In view of numerous biological properties associated with 1,4-DHP and the biological importance of the oxidation step of 1,4-DHP [20] , we became interested in the synthesis of some new 1,4-DHPs and their corresponding pyridine derivatives via iodine (III) mediated oxidation. In continuation of our work on the utility of iodine (III) reagents for the synthesis of various types of heterocyclic compounds, we herein carried out the synthesis of 4-arylpyridines using IBD as a greener oxidant at room temperature, ultrasonic and microwave irradiation.
2. Result and discussion
The present manuscript reports the synthesis of targeted diethyl 2,6-dimethyl-4-aryl-pyridine-3,5-dicarboxylate (4a-4m), by a one pot domino process. The main aim of this manuscript is to develop efficient synthetic methodology which requires lesser reaction time and reduces the number of steps involved in the synthesis of 4a-4m. In order to achieve our aim, synthesis of diethyl 2,6-dimethyl-4-((1,3-diphenyl)-1H-pyrazol-4-yl) pyridine-3,5-di- carboxylate was carried out by a two-step reaction. We first synthesized diethyl 2,6-dimethyl-4-((1,3-diphenyl)- 1H-pyrazol-4-yl) pyridine-3,5-dicarboxylate (3a). For this a pseudo four component reaction of ethyl acetoacetate (1) (2.0 mmol), 1,3-diphenyl-1H-pyrazole-4-carbaldehyde (2) (1.0 mmol) and ammonium acetate (2.2 mmol) was carried out in ethanol and the reaction mixture was allowed to reflux for 25 - 35 min. The progress of the reaction was checked by TLC using petroleum ether: ethyl acetate (85: 15, v/v) as eluent. After completion of the reaction as evident from TLC, the reaction mixture was cooled down to room temperature and the solid separated was filtered under suction to afford 2,6-dimethyl-4-((1,3-diphenyl)-1H-pyrazol-4-yl)-1,4-di-hydropyridine-3,5-dicar- boxylate (1a) as the desired product in 75% yield. The product so obtained was subjected to oxidation using IBD (1.2 mmol) in DCM at room temperature. The reaction was completed in 5 min as evident from TLC (petroleum ether: ethyl acetate (85: 15, v/v)). After the completion of reaction as evident from TLC, the reaction mixture was washed with aqueous NaHCO3 solution. Organic phase was then separated, dried and concentrated on water bath. Crude product, thus obtained, was purified by silica gel column chromatography (petroleum ether: ethyl acetate, 97:3, v/v) to afford diethyl 2,6-dimethyl-4-((1,3-diphenyl)-1H-pyrazol-4-yl) pyridine-3,5-dicarboxylate (4a) as a pure product in 68% yield. In order to improve the overall yield of 4a and to reduce the time required, this method can be further expanded to a modular one-pot synthesis, without isolation of intermediate 3a, in contrast to our previous protocols. We decided to try a domino process for the synthesis of 4a without the isolation of 3a in one pot. To achieve this, firstly a mixture of ethyl acetoacetate (1) (2.0 mmol), 1, 3-diphenyl-1H-pyrazole-4-carbal- dehyde (2) (1.0 mmol) and ammonium acetate (2.2 mmol) was taken in 50 mL round bottomed flask using acetonitrile as solvent. The content of the flask was heated to reflux for 20 - 25 min. The progress of the reaction was monitored by TLC using petroleum ether: ethyl acetate (85: 15, v/v) as eluent. After formation of 3a as confirmed by TLC, IBD (1.2 mmol) was then added to the same reaction mixture and the reaction mixture was refluxed again for 5 - 8 min. After the completion of reaction as evident from TLC, the reaction mixture was cooled at room temperature and washed with aqueous NaHCO3 solution. Organic phase was then separated, dried and concentrated on water bath. Crude product, thus obtained, was purified by silica gel column chromatography to afford pure diethyl 2,6-dimethyl-4-((1,3-diphenyl)-1H-pyrazol-4-yl)pyridine-3,5-dicarboxylate (4a) in 85% yield. The generality of the optimized protocol was checked by carrying out the reactions using different 3-(aryl)-1- phenyl-1H-pyrazole-4-carbaldehyde and also substituted benzaldehydes containing both electron withdrawing and electron releasing substituents yielded the corresponding diethyl 2,6-dimethyl-4-arylpyridine-3,5-dicarbox- ylate (4a-4m) in excellent yields. (Scheme 1, Table 1).
The efficiencies of this protocol prompted us to explore this protocol further using Ultrasonic and microwave irradiation to reduce the present serious energy crisis in the environment. All the reactions proceeded successfully and yielded the corresponding products in excellent yield. The results obtained using ultrasonic and microwave irradiation is summarized in Table 1.
3. Conclusion
We have synthesized a series of 2,6-dimethyl-4-aryl-pyridine-3,5-dicarboxylate derivatives (4a-4m) by one-pot domino process in acetonitrile and IBD as a greener oxidant using conventional heating, ultrasonic and microwave irradiation. This method thus provides a one pot, facile, rapid and efficient synthesis of compounds 4a-4m which are otherwise accessible through a two-step process.
4. Experimental
Structures of all the compounds were identified by their spectral data. Silica gel 60 F254 (Precoated aluminium plates) from Merck were used to monitor reaction progress. Melting points were determined on a melting point apparatus and are uncorrected. IR (KBr) spectra were recorded on buck scientific IR M-500 spectrophotometer and the values are expressed as νmax cm−1. The 1H NMR spectra were scanned on a Bruker (300 MHz) spectrometer in CDCl3 using tetramethylsilane as an internal standard. Mass spectral data were recorded on a Waters micromass Spectrometer running under Mass Lynex version 4.0 software and equipped with an ESI source. The chemical shift values are recorded on δ scale and the coupling constants (J) are in Hz. Ultrasonic bath (54 KHz, 300 W, 1 Lt, capacity) of Through clean ultrasonic Pvt. Ltd. (India) was used for reactions under ultrasonic irradiation. CEM discover microwave reactor was used for reactions under microwave irradiation. Pyrazole aldehydes (3) were synthesized according to the literature method [21] .
4.1. Preparation of (4a-4m) under Conventional heating
A mixture of ethylacetoacetate (1) (1.0 mmol), aryl aldehyde (2) (1.0 mmol), and ammonium acetate (2.2 mmol) was dissolved in 5 mL of acetonitrile in a 50 mL round-bottomed flask. The reaction contents were refluxed on water bath for 20 - 25 min. The progress of the reaction was monitored by TLC using petroleum ether: ethyl acetate (85: 15, v/v) as eluent. After formation of 3a as evident from TLC after 25 min, IBD (1.2 mmol) was then added to the above reaction mixture and the reaction mixture was refluxed for another 5 - 8 min. After consumption of 3a as evident from TLC, the reaction mixture was cooled to room temperature and washed with aqueous NaHCO3 solution. Organic phase was then separated, dried and concentrated on water bath. Crude product, thus obtained, was purified by silica gel column chromatography to afford pure diethyl 2,6-dimethyl- 4-aryl-pyridine-3, 5-dicarboxylate (4a-4m). All the compounds 4a-4m are characterized by 1H NMR, 13C NMR, IR and mass data.
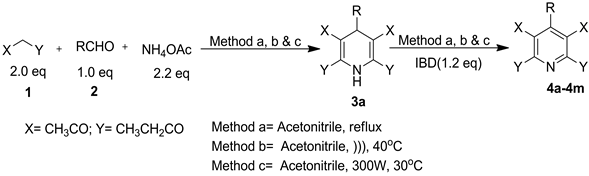
Scheme 1. Synthesis of diethyl 2,6-dimethyl-4-aryl-pyridine-3,5-dicarboxy- late (4a-4m) using one pot domino protocol.
![]()
Table 1. Synthesis of diethyl 2,6-dimethyl-4-aryl-pyridine-3,5-dicarboxylate (4a-4m) using conventional heating, ultrasonic and microwave irradiation.
aIsolated yield.
4.2. Preparation of (4a-4m) under ultrasonic irradiation
A mixture of ethylacetoacetate (1) (1.0 mmol), aryl aldehyde (2) (1.0 mmol), and ammonium acetate (2.2 mmol) was dissolved in 5 mL of acetonitrile in a 50 mL round-bottomed flask. The reaction contents were sonicated at 40˚C for appropriate time as mentioned in Table 1. The progress of the reaction was monitored by TLC using petroleum ether: ethyl acetate (85: 15, v/v) as eluent. After formation of 3a as evident from TLC after 8 min, IBD (1.2 mmol) was then added to the above reaction mixture and the reaction mixture sonicated at room temperature for another 3 - 4 min. After consumption of 3a as evident from TLC the reaction mixture was washed with aqueous NaHCO3 solution. Organic phase was then separated, dried and concentrated on water bath. Crude product, thus obtained, was purified by silica gel column chromatography to afford pure diethyl 2,6-dimethyl-4-aryl- pyridine-3,5-dicarboxylate (4a-4m). All the compounds 4a-4m are characterized by 1H NMR, 13C NMR, IR and mass data.
4.3. Preparation of (4a-4m) under microwave irradiation
A mixture of ethylacetoacetate (1) (1.0 mmol), aldehyde (2) (1.0 mmol), and ammonium acetate (2.2 mmol) was dissolved in 5 mL of acetonitrile in a sealed vial and placed in a CEM Discover microwave reactor. The vial was subjected to microwave irradiation, programmed at 30˚C and 300 W. After formation of 3a as evident from TLC after 1 min, IBD (1.2 mmol) was then added to the above reaction mixture and the reaction mixture irradiated for another 30 sec. After completion of the reaction as evident from TLC, the reaction mixture was washed with aqueous NaHCO3 solution. Organic phase was then separated, dried and concentrated on water bath. Crude product, thus obtained, was purified by silica gel column chromatography to afford pure diethyl 2,6-dimethyl-4-aryl- pyridine-3,5-dicarboxylate (4a-4m). All the compounds 4a-4m are characterized by 1H NMR, 13C NMR, IR and mass data.
4.4. Characterization Data
4.4.1. 4-(1,3-Diphenyl-1H-pyrazol-4-yl)-2,6-Dimethyl-pyridine-3,5-dicarboxylic Acid Diethyl Ester (4a)
Mp: 111˚C; IR (νmax, cm−1, KBr): 1736, 1233; 1H NMR (300 MHz, CDCl3, δ, ppm): 0.95 (t, 6H, CH3), 2.613 (s, 6H, CH3), 3.910 - 4.07 (m, 4H, OCH2), 7.110 - 7.313 (m, 4H), 7.817 (s, 1H), 7.581 - 7.690 (m, 6H); 13C NMR (75 MHz, DMSO-d6) 166.8, 155.7, 147.8, 139.3, 138.2, 133.4, 131.2, 130.2, 129.2, 128.8, 128.0, 127.2, 118.5, 115.7, 61.7, 23.0, 13.5; Elemental analysis: Calcd for C28H27N3O4: C 71.64, H 5.76, N 8.95; found: C 71.63, H 5.79, N 8.93; MS (m/z): 470.20 (M+ + 1).
4.4.2. 2,6-Dimethyl-4-(1-phenyl-3-P-tolyl-1H-pyrazol-4-yl)-Pyridine-3,5-dicarboxylic acid Diethyl ester (4b)
Mp: 105˚C; IR (νmax, cm−1, KBr): 1720, 1234; 1H NMR (300 MHz, CDCl3, δ, ppm): 0.93 (t, 6H, CH3), 2.611 (s, 6H, CH3), 3.810 (s, 3H), 3.99 (q, 4H, OCH2), 6.84 (d, 2H, J = 8.7 Hz), 7.280 - 7.501 (m, 5H), 7.732 - 7.759 (d, 2H, J = 8.7 Hz), 7.905 (s, 1H); 13C NMR (75 MHz, DMSO-d6); 167.2, 155.7, 147.9, 139.5, 137.9, 137.2, 129.8, 128.3, 127.9, 127.6, 127.3, 127.1, 119.2, 116.2, 61.2, 34.4, 23.1, 13.4; Elemental analysis: Calcd for C29H29N3O4: C 72.05, H 6.00, N 8.70; found: C 72.06, H 6.05, N 8.70; MS (m/z): 484.40 (M+ + 1).
4.4.3. 4-[3-(4-Methoxy-phenyl)-1-phenyl-1H-pyrazol-4-yl]-2,6-dimethyl-pyridine-3,5-Dicarboxylic Acid diethyl Ester (4c)
Mp: 136˚C; IR (νmax, cm−1, KBr): 1740, 1034; 1H NMR (300 MHz, CDCl3, δ, ppm): 0.95 (t, 6H, CH3), 2.612 (s, 6H, CH3), 3.808 (s, 3H), 4.00 (q, 4H, OCH2), 6.84 (d, 2H, J = 8.7 Hz), 7.311−7.501 (m, 5H), 7.74 (d, 2H, J = 8.7 Hz), 7.905 (s, 1H); 13C NMR (75 MHz, DMSO-d6); 167.1, 155.9, 148.5, 139.6, 137.8, 137.3, 129.6, 128.4, 127.9, 127.8, 127.4, 127.2, 119.0, 116.3, 61.4, 44.4, 23.0, 13.5; Elemental analysis: Calcd for C29H29N3O5: C 69.73, H 5.81, N 8.41; found: C 69.71, H 5.83, N 8.40; MS (m/z): 500.29 (M+ + 1).
4.4.4. 4-[3-(4-Fluoro-Phenyl)-1-phenyl-1H-pyrazol-4-Yl]-2,6-dimethyl-pyridine-3,5-Dicarboxylic Acid diethyl Ester (4d)
Mp: 121˚C; IR (νmax, cm−1, KBr): 1728, 1236, 1037; 1H NMR (300 MHz, CDCl3, δ, ppm): 0.94 (t, 6H, CH3), 2.615 (s, 6H, CH3), 3.905 - 4.105 (q, 4H, OCH2), 6.987 - 7.044 (m, 2H), 7.280 - 7.365 (m, 1H), 7.469 - 7.622 (m, 4H), 7.74 (d, 2H, J = 7.8 Hz), 7.923 (s, 1H); 13C NMR (75 MHz, DMSO-d6) 166.9, 155.6, 148.9, 139.4, 138.1, 133.3, 131.3, 130.1, 129.0, 128.9, 128.0, 127.2, 118.6, 115.8, 61.6, 23.1, 13.6; Elemental analysis: Calcd for C28H26N3O4F: C 68.99, H 5.38, N 8.62; found: C 68.95, H 5.37, N 8.63; MS (m/z): 488.36 (M+ + 1).
4.4.5. 4-[3-(4-Chlorophenyl)-1-phenyl-1H-Pyrazol-4-yl]-2,6-dimethyl-Pyridine-3,5-dicarboxylic Acid diethyl Ester (4e)
Mp: 101˚C - 102˚C (101˚C - 102˚C, lit [22] ); IR (νmax, cm−1, KBr): 3055, 2989, 1747, 1620, 1597, 1461, 1322, 1087, 1002, 952, 850, 836, 698; 1H NMR (300 MHz, CDCl3, δ, ppm): 0.935 (t, J = 7.2 Hz, 6H, CH3), 2.618 (s, 6H, CH3), 3.898 - 4.118 (m, 4H, OCH2), 7.310 - 7.370 (m, 2H); 7.487 - 7.513 (m, 5H), 7.746 (d, J = 7.8 Hz, 2H), 7.923 (s, 1H); Elemental analysis: Calcd. for C28H26ClN3O4: C, 66.73; H, 5.20; N, 8.34. Found: C, 61.66; H, 5.29; N, 8.26.
4.4.6. 4-[3-(4-Bromo-phenyl)-1-phenyl-1H-pyrazol-4-Yl]-2,6-dimethyl--pyridine-3,5-Dicarboxylic Acid diethyl Ester (4f)
Mp: 115˚C; IR (νmax, cm−1, KBr): 1734, 1030; 1H NMR (300 MHz, CDCl3, δ, ppm): 0.95 (t, 6H, CH3), 2.617 (s, 6H, CH3), 3.99 (q, 4H, OCH2), 7.200 - 7.495 (m, 7H), 7.74 (d, 2H, J = 7.2 Hz), 7.921 (s, 1H); 13C NMR (75 MHz, DMSO-d6) 166.5, 155.7, 148.8, 139.6, 138.1, 132.3, 131.2, 130.0, 129.1, 128.8, 128.0, 127.1, 117.6, 115.6, 61.7, 23.4, 13.5; Elemental analysis: Calcd for C28H26N3O4Br: C 61.42, H 4.75, N 7.68; found: C 61.31, H 4.79, N 7.69. MS (m/z): 548.20, 550.20.
4.4.7. 2,6-Dimethyl-4-[3-(4-nitro-Phenyl)-1-phenyl-1H-pyrazol-4-Yl]-pyridine-3, 5-Dicarboxylicacid diethyl ester (4g)
Mp: 172˚C; IR (νmax, cm−1, KBr): 1728, 1234, 1034; 1H NMR (300 MHz, CDCl3, δ, ppm): 0.91 (t, 6H, CH3), 2.632 (s, 6H, CH3), 3.923 - 4.039 (m, 4H, OCH2), 7.279 - 7.410 (m, 3H), 7.499 - 7.769 (m, 4H), 7.960 (s, 1H), 8.19 (d, 2H, J = 7.5 Hz); 13C NMR (75 MHz, DMSO-d6) 167.9, 155.8, 149.0, 139.8, 138.2, 133.6, 131.5, 130.3, 129.2, 128.9, 128.0, 127.4, 119.6, 116.6, 62.5, 23.4, 13.7; Elemental analysis: Calcd for C28H26N4O6: C 64.37, H 4.98, N 10.73; found: C 65.34, H 5.08, N 10.87; MS (m/z): 515.26 (M+ + 1).
4.4.8. Diethyl-4-phenyl-2,6−dimethylpyridine-3,5-Dicarb Oxylate (4h)
Mp: 62˚C - 63˚C; IR (νmax, cm−1, KBr): 3026, 2978, 1729, 1592, 1477, 1301, 1212, 1171, 792, 761; 1H NMR (300 MHz, CDCl3, δ, ppm): 1.22 (t, J = 7.11 Hz, 6H, CH3), 4.27 (q, J = 7.11 Hz, 4H, OCH2), 2.67 (s, 6H, CH3), 7.18 - 7.23 (m, 2H), 7.30 - 7.32 (m, 3H); Elemental analysis: Calcd. for C19H21NO4: C, 69.71; H, 6.47; N, 4.28. Found: C, 69.88; H, 6.55; N, 4.19.
4.4.9. Diethyl-4-(4-methylphenyl)-2,6-dimethylpyridine-3,5-dicarboxylate (4i)
Mp: 70˚C - 71˚C (71˚C -72˚C, lit [22] ); IR (νmax, cm−1, KBr): 3022, 2978, 1725, 1582, 1444, 1228, 1013, 822, 857, 776 ; 1H NMR (300MHz, CDCl3, δ, ppm): 1.234 (t, J = 7.11 Hz, 6H, CH3), 2.35 (s, 3H, CH3), 2.66 (s, 6H, CH3), 4.28 (q, J = 7.11 Hz, 4H, OCH2), 7.12 (d, J = 6.79 Hz, 2H), 7.23 (d, J = 6.79 Hz, 2H); Elemental analysis: Calcd. for C20H23NO4: C, 70.36; H, 6.79; N, 4.10. Found: C, 70.44; H, 6.84; N, 4.28.
4.4.10. Diethyl-4-(3-bromophenyl)-2,6-dimethylpyr-idine-3,5-Dicarboxylate (4j)
Mp: 71˚C - 73˚C (70˚C - 72˚C, lit [22] ); IR (νmax, cm−1, KBr): 3055, 2988, 1727, 1562, 1280, 1102, 1035, 866, 777, 697; 1H NMR (300MHz, CDCl3, δ, ppm): 1.24 (t, J = 7.13 Hz, 6H, CH3), 4.30 (q, J = 7.13 Hz, 4H, OCH2), 2.66 (s, 6H, CH3), 7.20 - 7.44 (m, 4H); Elemental analysis: Calcd. for C19H20BrNO4 C, 56.17; H, 4.96; N, 3.45. Found: C, 56.32; H, 4.88; N, 3.28.
4.4.11. Diethyl-4-(4-chlorophenyl)-2,6-dimethylpyr-idine-3,5-Dicarboxylate (4k)
Mp: 70˚C - 72˚C (69˚C - 71˚C, lit [22] ); IR (νmax, cm−1, KBr): 3028, 2991, 1728, 1588, 1232, 1106, 1045, 857, 657; 1H NMR (300MHz, CDCl3, δ, ppm): 1.22 (t, J = 7.11 Hz, 6H, CH3), 4.27 (q, J = 7.11 Hz, 4H, OCH2), 2.70 (s, 6H, CH3), 7.12 (d, J = 8.99 Hz, 2H), 7.32 (d, J = 8.99 Hz, 2H). Elemental analysis: Calcd. for C19H20ClNO4: C, 63.07; H, 5.57; N, 3.87. Found: C, 62.92; H, 5.66; N, 3.66.
4.4.12. Diethyl-4-(4-methoxyphenyl)-2,6-dimethyl pyridine-3,5-Dicarboxylate (4l)
Mp:50˚C - 51˚C (51˚C - 52˚C, lit [22] ); IR (νmax, cm−1, KBr): 3034, 2987, 1731, 1599, 1523, 1288, 1107, 856, 834, 772; 1H NMR (300MHz, CDCl3, δ, ppm): d = 1.22 (t, J = 7.12 Hz, 6H, CH3), 4.27 (q, J = 7.12 Hz, 4H, OCH2), 2.69 (s, 6H, CH3), 3.86 (s, 3H, OCH3), 6.91 (d, J = 8.57 Hz, 2H), 7.11 (d, J = 8.57 Hz, 2H); Elemental analysis: Calcd. for C20H23NO5: C, 67.21; H, 6.49; N, 3.92. Found: C, 67.34; H, 6.54; N, 4.02.
4.4.13. Diethyl-4-(4-nitrophenyl)-2,6-dimethylpyri-dine-3,5-Dicarboxylate (4m)
Mp: 110˚C - 112˚C (112˚C - 113˚C, lit [22] ); IR (νmax, cm−1, KBr): 3023, 2988, 1726, 1555, 1504, 1351, 1106, 866, 843, 745; 1H NMR (300MHz, CDCl3, δ, ppm):1.23 (t, J = 7.12 Hz, 6H, CH3), 2.63(s, 6H, CH3), 4.25 (q, J = 7.12 Hz, 4H, OCH2), 7.41 (d, J = 8.23 Hz, 2H), 8.22 (d, J = 8.23 Hz, 2H). Elemental analysis: Calcd. for C19H20N2O6: C, 61.29; H, 5.41; N, 7.53. Found: C, 61.44; H, 5.32; N, 7.65.
Acknowledgements
We are thankful to CSIR, New Delhi for the award of Junior Research Fellowship (JRF) to Khalid Hussain and KUK for Teaching Assistantship to Deepak Wadhwa.
NOTES
*Corresponding author.