Randomized, Placebo-Controlled Trial of Transdermal Rivastigmine for the Treatment of Encephalopathy in Liver Cirrhosis (TREC Trial) ()
1. Introduction
Hepatic encephalopathy (HE) is a neuropsychiatric complication of acute and chronic liver disease [1] that contributes to the mortality of patients with end-stage liver disease [2] . This potentially reversible condition [3] is characterized by a variety of symptoms, including changes in consciousness and cognition [4] . The precise pathogenic mechanisms leading to HE are unknown but are primarily believed to involve the accumulation of ammonia and other gut-derived toxins [5] . Such current approaches to the management of HE are primarily designed to reduce the levels of ammonia and other gut-derived toxins using therapies such as non-absorbable disaccharides (e.g., lactulose) and non-systemic antibiotics (e.g., rifaximin) [6] .
Accumulation of ammonia and gut-derived toxins can lead to a variety of pathogenic changes, including alterations in neurotransmitter signaling systems [3] [6] . While the changes in neurotransmitter systems may be complex and heterogenous, [7] upregulation of the inhibitory GABAergic and serotonergic pathways and impairment of the excitatory glutamatergic and catecholaminergic pathways have been hypothesized to be involved in the pathogenesis of HE [6] . Alterations in the cholinergic neurotransmitter system have also been observed in both animal models of HE [8] -[12] and in postmortem brain tissue samples from patients with HE [7] [8] [13] . For example, the level of acetylcholine (ACh) and the neurotransmitter of the cholinergic system, were reduced, and the activity of acetylcholinesterase (AChE), the enzyme that hydrolyzes ACh, was increased in brain extracts from rats with experimentally induced HE [8] . Similarly, the activity of AChE was higher in postmortem brain samples from patients with HE compared with controls without cirrhosis [8] . Given the importance of cholinergic neurotransmission in cognitive function and consciousness [14] alterations such as those, which disrupt cholinergic signaling, may explain the deficits in cognitive function and consciousness observed in patients with HE. Thus, therapies that may restore cholinergic balance may be useful in the treatment of patients with HE.
Rivastigmine is a reversible AChE inhibitor used to treat dementia associated with Alzheimer’s disease (AD) and Parkinson’s disease [15] . Rivastigmine significantly improved learning in a rat model of HE [8] , but, to date, there have been no reports of rivastigmine improving cognitive function in patients with HE. However, case reports of patients with HE treated with the AChE inhibitors neostigmine (with polyethylene glycol [PEG]) [16] [17] and physostigmine [18] have suggested that this class of drugs may be effective for the treatment of HE in humans. The objective of this randomized, placebo-controlled pilot study was to determine the efficacy and safety of transdermal rivastigmine for improving cognitive function in patients with grade 2 or 3 HE.
2. Methods
This was a randomized, placebo-controlled pilot study conducted between 2009 and 2010. The protocol was approved by the institutional review board at Finestein Institute. The study was performed in accordance with the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice Guidelines, and applicable local laws and regulations. Signed informed consent was obtained for each patient before study enrollment.
2.1. Patient Population
Patients were eligible if they had grade 2 or 3 HE. Hepatic encephalopathy grades were based on clinical and electroencephalographic criteria [19] [20] . Grade 2 HE was characterized by the presence of lethargy, inappropriate behavior, disorientation, asterixis, abnormal reflexes, and abnormalities on electroencephalogram (EEG) (i.e., slowing, triphasic waves). Patients with grade 3 HE exhibited somnolence (but were rousable), loss of meaningful communication, asterixis, abnormal reflexes, and slowing, triphasic waves on EEG. Exclusion criteria included the recent or current use of antihistamines, metoclopramide, benzodiazepines, cannabinoids, or narcotics; acute gastrointestinal bleeding; sepsis; toxic metabolic syndrome; renal failure; and infection with human immunodeficiency virus. Urine was screened before the study and then weekly to detect the use of narcotics, benzodiazepines, and cannabinoids.
2.2. Treatments
Eligible patients were treated with oral lactulose (20 g/30 mL three times daily) and randomized to receive either the transdermal rivastigmine patch (Exelon® Patch; Novartis Pharmaceuticals Corporation, East Hanover, NJ; 4.6 mg/d) or a placebo patch for 21 days. Randomization was performed by electronic randomization. Dietary animal protein intake was restricted to 50 g/d. The use of oral or intravenous antibiotics was prohibited during the 21 days of the study.
2.3. Assessments
Hepatic encephalopathy was assessed using the modified encephalopathy score (MES), a measure of memory loss, confusion, sleep disturbance, and comprehension with a minimum score of 4 (mild HE) and a maximum score of 12 (severe HE; Table 1). All questions for the MES were administered in the patient’s native language. Psychometric testing was performed using the trail test (TT) [21] and the object recognition test (ORT). The MES, TT, ORT, and serum ammonia levels were assessed at baseline and then weekly. Electroencephalography was performed at baseline and during the final week of the study. Safety was assessed
2.4. Statistical Analysis
T-test was used to compare transdermal rivastigmine to placebo by calculating p values for statistical significance (defined as P ≤ 0.05) using SPSS version 20 for mac.
3. Results
3.1. Patients and Baseline Characteristics
A total of 72 patients were screened (Figure 1). Of 12 who did not participate 8 did not meet the inclusion criteria and 4 declined to participate after signing the consent. Thirty patients were randomized; 15 received the transdermal rivastigmine patch and 15 received a placebo patch.
The demographics of the treatment groups were similar with respect to age, sex, and body mass index (Table 2). There were more black patients and fewer white and Hispanic patients in the transdermal rivastigmine group

Table 1 . Modified encephalopathy scoring systema.
aPatients are rated from 1 to 3 for each variable, and scores are added to calculate the modified encephalopathy score. Total score can range from 4 (mild) to 12 (severe).
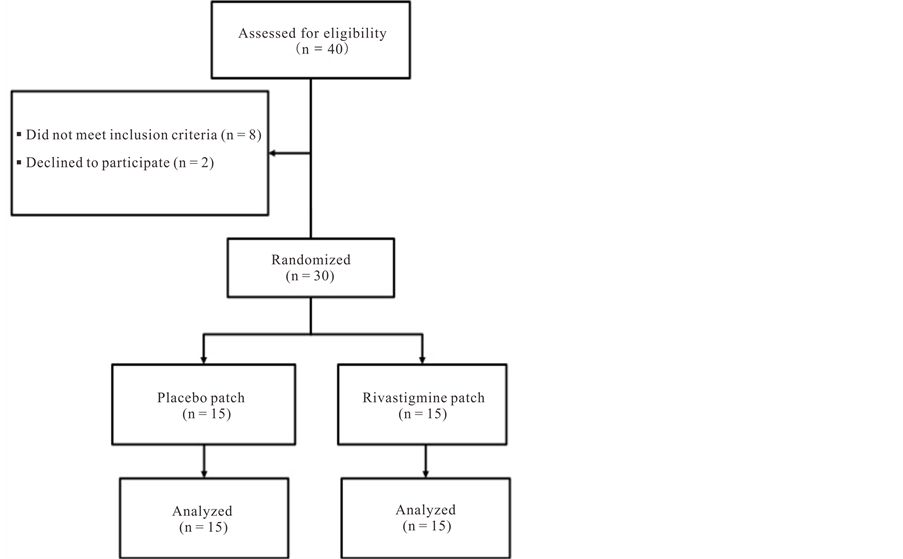
Figure 1. Patient disposition.
than in the placebo group. Baseline disease characteristics were similar between the treatment groups with respect to model for end-stage liver disease score, EEG, MES, and performance on the TT and ORT (Table 2). More patients in the placebo group had alcoholic liver disease (40%) compared with the transdermal rivastigmine group (13%). Serum ammonia levels were slightly but significantly higher in the transdermal rivastigmine group compared with the placebo group (P = 0.03).
3.2. Modified Encephalopathy Score
Transdermal rivastigmine resulted in a significantly lower mean MES compared with placebo within 1 week of treatment (7 vs 10, respectively; P < 0.0001; Figure 2). Though the mean MES of both treatment groups leveled off during weeks 2 and 3, the significant effect of transdermal rivastigmine compared with placebo on the mean MES was sustained at weeks 2 and 3 of the study (P < 0.0001 at both weeks). These results suggest that transdermal rivastigmine was significantly more effective than placebo at improving the degree of cognitive dysfunction in HE in patients receiving concomitant lactulose.
3.3. Psychometric Tests
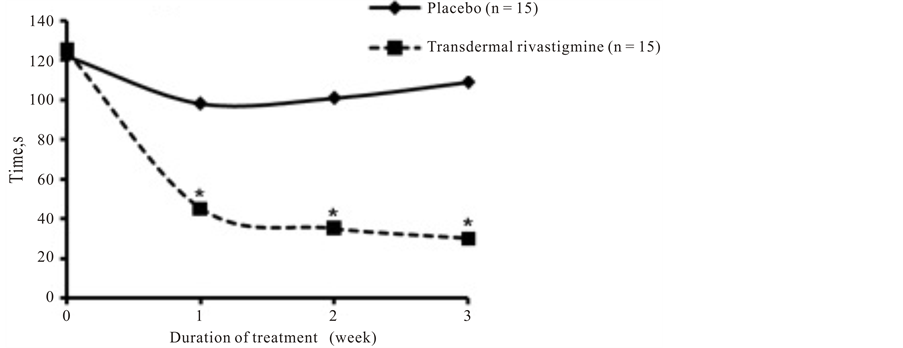
Patients who received transdermal rivastigmine had significantly better performance on both the ORT (Figure 3(a)) and TT (Figure 3(b)) during weeks 1 through 3 of the study compared with those who received placebo (P = 0.0001 at all 3 time points for both tests). In patients treated with transdermal rivastigmine, ORT results (45 seconds) were normal (<55 seconds) within 1 week of treatment with transdermal rivastigmine. The improvement in the ORT with transdermal rivastigmine was sustained as scores remained normal during weeks 2 and 3 of treatment (35 and 30 seconds, respectively). In contrast, in patients who received placebo, ORT results only slightly improved from 125 seconds at baseline to 98 seconds after 1 week and actually worsened during weeks 2 and 3 of the study (101 and 109 seconds, respectively).
Results of the TT were also substantially improved in patients who received transdermal rivastigmine, decreasing from a baseline score of 161 seconds to 90 seconds and 92 seconds after 1 and 2 weeks of treatment, respectively. By the third week of treatment, TT results (65 seconds) approached normal (<60 seconds). In contrast, TT results in the placebo group only slightly improved from 158 seconds at baseline to 154, 134, and 146

Table 2 . Patient demographics and baseline disease characteristics.
BMI, body mass index; EEG, electroencephalogram; HE, hepatic encephalopathy; MELD, model for end-stage liver disease; MES, modified encephalopathy score; ORT, object recognition test; TT, trail test. aNormal range, 11 - 32 mol/L. bP = 0.03. cNormal, <55 seconds. dNormal, <60 seconds.
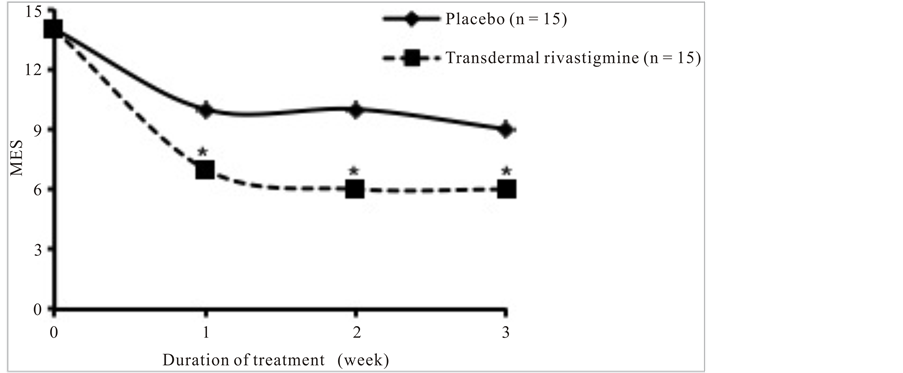
Figure 2. Modified encephalopathy scores over the 3 weeks of treatment with placebo or transdermal rivastigmine. *P < 0.0001. MES, modified encephalopathy score.
seconds after 1, 2, and 3 weeks of treatment, respectively.
3.4. Clinical Tests
For patients in both treatment groups, serum ammonia levels were progressively lower each of the 3 weeks of
 (a)
(a)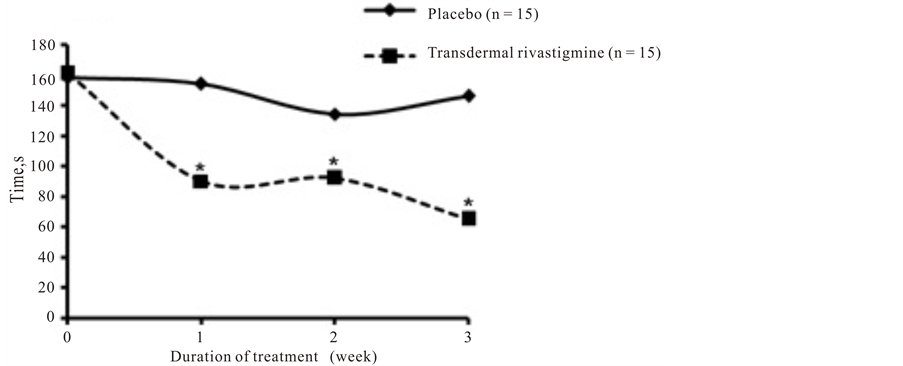 (b)
(b)
Figure 3. Psychometric tests. Scores for (a) object recognition test and (b) trail test over the 3 weeks of treatment with placebo or transdermal rivastigmine. Normal scores for object recognition test and trail testare less than 55 seconds and less than 60 seconds, respectively.*P = 0.0001.
the study compared with baseline (Table 3). However, transdermal rivastigmine did not have a greater effect on serum ammonia levels than placebo. In fact, patients who received placebo had slightly but significantly greater reductions in serum ammonia levels compared with those who received transdermal rivastigmine after 2 weeks of treatment (P = 0.03). However, by the third week of treatment, thereduction in serum ammonia was not significantly different between the 2 groups. There were no differences in EEG results between the 2 treatment groups.
3.5. Safety
Overall, transdermal rivastigmine was well tolerated, and no reactions were reported at the patch-application site. In the transdermal rivastigmine group, 1 patient (7%) reported diarrhea and 1 (7%) reported dry mouth. In the placebo group, 2 patients (13%) reported diarrhea, 1 (7%) reported dry mouth, and 1 (7%) reported urinary retention (Table 4).
4. Discussion
The results of this randomized, placebo-controlled pilot study suggest that transdermal rivastigmine significantly improves cognitive function compared with placebo in patients with grade 2 or 3 HE who were receiving con

Table 3. Serum ammonia levels.
NS = not significant.
improves cognitive function compared with placebo in patients with grade 2 or 3 HE who were receiving concomitant lactulose. These results support those of earlier case reports in which hepatic coma resolved in 3 patients with HE who were treated with other AChE inhibitors [16] -[18] . In 2 of the patients, treatment with neostigmine and PEG electrolyte solution to improve gastric motility and bowel cleansing, respectively, resulted in the resolution of hepatic coma after 2 days [16] [17] . The third patient was treated with physostigmine and immediately regained consciousness after administration of the AChE inhibitor [18] .
The observation that inhibition of AChE enhances cognitive function is not unique to HE. Rivastigmine is also used to treat AD and Parkinson’s disease dementia. The AChE inhibitors galantamine and donepezil are also used for the treatment of AD. Inhibition of AChE may improve cognitive function in patients with HE, AD, or Parkinson’s disease dementia by increasing levels of ACh in the brain and prolonging ACh activity at neural synapses [15] . However, there is mounting evidence that AChE inhibitors may have an antiinflammatory effect [22] which has been observed in patients with AD [23] . Such a mechanism may be relevant to patients with HE because cerebral inflammation has been directly implicated in the pathogenesis of HE in a rat model of acute liver failure, [24] and systemic inflammation has been implicated in the pathogenesis of HE [25] and the impairment of neuropsychologic function [26] in patients with liver disease. In patients with HE, AChE inhibitors may also increase gastrointestinal motility and thereby enhance the effect of intestinal cathartics such as lactulose and PEG, which were used concomitantly in this study and the previously described case reports, respectively [16] -[18] .
While rivastigmine is available in oral and transdermal formulations, the transdermal formulation may be more favorable in general and for patients with HE. First, transdermal rivastigmine generally offers improved tolerability compared with the oral formulation, particularly with respect to gastrointestinal adverse events such as nausea and vomiting [15] [27] . This may be because of the more gradual and smoother increase in absorption of transdermal rivastigmine compared with oral rivastigmine [15] . Second, the ease of dosing of the transdermal patch compared with oral formulations may be advantageous in patients with HE, particularly those with higher grades of HE who may be unable to easily ingest tablets or oral solutions. Lastly, oral rivastigmine is administered twice daily [28] while the patch is applied only once daily [29] . This dosing schedule may provide less interference with daily life. Overall, the advantages of transdermal delivery of rivastigmine may increase patient compliance and satisfaction for patients and caregivers. Indeed, caregivers of patients with AD preferred the transdermal rivastigmine patch compared with the oral capsule in a large (n = 1059 caregivers) 24-week study comparing the transdermal and oral formulations of rivastigmine in patients with AD [30] .
One limitation to this study is that it was performed using a small population of patients (N = 30). Furthermore, patients in both treatment groups were receiving concomitant lactulose. The finding in this study that there was improvement of MES in the placebo group (albeit to a significantly lesser degree than the transdermal rivastigmine group) suggests that lactulose may have contributed to the improvement in MES observed in this study. However, patients who received placebo did not experience any substantial or sustained benefit in cognitive function as assessed using the TT and ORT. This suggests that transdermal rivastigmine had an independent effect on cognitive function in patients with grade 2 or 3 HE.
Interestingly, despite the improvement in cognitive function with transdermal rivastigmine, there was no improvement in serum ammonia levels with the AChE inhibitor compared with placebo. In fact, after 2 weeks of treatment, serum ammonia levels were reduced to a slightly but significantly greater degree in patients who received placebo compared with those who received transdermal rivastigmine. This suggests that reductions in serum ammonia levels are not clinically relevant in the context of improving the results of psychometric tests such as the TT and ORT. It is notable that serum ammonia levels can be influenced by several factors, including the conditions leading to HE (i.e., precipitating factors), mild exertion by the patient before phlebotomy (including fist clenching), prolonged tourniquet application, and blood-sample drawing technique andstorage [1] [6] [31] -[33] . Thus, results may not be comparable among patients or even between samples taken at different times from an individual patient.
In this study, transdermal rivastigmine with lactulose was safe and well tolerated. Diarrhea, a common adverse event associated with lactulose treatment, [6] was reported by 1 patient who received transdermal rivastigmine and 2 patients who received placebo. Adverse events associated with AChE-inhibitor use were only reported by 1 patient in the transdermal rivastigmine group (dry mouth) and by 2 patients in the placebo group (dry mouth, urinary retention). Further, no skin irritation at the patch-attachment site was reported in this study. The favorable safety and tolerability profile reported here for transdermal rivastigmine in patients with HE is comparable to that reported in patients with AD, although a small percentage of patients with AD did develop some skin irritation [27] .
5. Conclusion
In conclusion, although performed with a small number of patients, this randomized, placebo-controlled pilot study demonstrates that transdermal rivastigmine used offlabel with concomitant lactuloseis is safe and effective for improving cognitive function in patients with HE. Large, randomized clinical trials are needed to fully explore the benefits of transdermal rivastigmine in patients with HE. Because of the benefit of transdermal rivastigmine on cognitive function shown here, these trials should be conducted not only in patients with overt HE (grade ≥ 1) but also in patients with minimal HE (grade 0) in which the only symptom is cognitive dysfunction measurable only by psychometric or neurophysiologic testing [6] .