1. Introduction
Zinc (Zn) deficiency is a widespread nutrient disorder in crop plants worldwide [1] [2] . The deficiency of micronutrients, including Zn, in crop plants is related to several soil and plant factors. These factors are low natural levels of micronutrients in the soils, use of high yielding cultivars, liming of acid soils, interactions among macro and micronutrients, planting on sandy and calcareous soils, increased use of high analysis fertilizers containing low amounts of micronutrients, and decreased use of organic amendments such as animal manures, composts, and crop residues [1] [2] . Graham [3] reported that half of the world’s soils are intrinsically deficient in Zn. Zinc deficiency in annual crops is reported in Brazil [4] , Australia [3] , India [5] , China [6] , Turkey [7] , Europe[8] , USA [9] , and Africa [10] .
In Brazil, Zn deficiency is very common in crop plants grown on Oxisols and Ultisols, which are acidic and have low concentrations of Zn [1] [4] [11] . In addition, these soils require liming to reduce soil acidity, improve Ca and Mg contents and increase activities of beneficial microorganisms. Zn deficiency in these soils has been reported in upland rice, corn, sorghum, wheat, soybean and dry bean [12] . In addition to Zn, these soils are also deficient in N, P, Ca, Mg, S, B and Cu [1] [13] [14] . The strategies which can be adopted to improve micronutrients uptake by crop plants can be divided into two groups. In the first group, bioavailability of soil micronutrients can be improved by adopting practices such as use of adequate rate, proper sources and suitable methods of application. Fertilizer management practices (source, rate, placement, and application time) should be optimized based on soil, plant, and climatic factors to reduce mineral losses (leaching, runoff, fixation) and improve their availability [1] . Improvement and consideration of these factors will enhance recovery of added fertilizers [1] . Cakmak [7] also reported that agronomic biofortification is of great importance in enriching the seeds of food crops with Zn. In the second group, use of crop species or genotypes within a species which are efficient in micronutrients uptake and translocation to the grain can improve Zn utilization efficiency [14] [15] .
Cover crops are important components of cropping systems in improving soil fertility, controlling weeds, diseases and insects [16] . In addition, cover crops also help in conserving soil moisture, reducing soil erosion and improving soil microbiology [16] [17] . Information is limited on the response of cover crops to Zn fertilization in acidic, infertile tropical soils of South America. Cover crop species having high Zn efficiency might produce higher yields and persist longer when grown on infertile soils where the supply of Zn is limited [18] [19] . The objectives of this study were to evaluate the influence of soil Zn levels on shoot and root growth and nutrition and use efficiency of Zn in ten important tropical legume cover crops.
2. Materials and Methods
A greenhouse experiment was conducted to determine Zn requirements of ten tropical legume crops. The soil used in the experiment was an Oxisol whose chemical and physical properties were: pH 5.1, Ca 0.2 cmolc·kg−1, Mg 0.2 cmolc·kg−1, Al 0.1 cmolc·kg−1, K 30 mg·kg−1, P 0.3 mg·kg−1, Zn 0.2 mg·kg−1, Cu 1.6 mg·kg−1, Fe 30 mg·kg−1, Mn 7 mg·kg−1, and organic matter 1.4 g·kg−1. Soil textural analysis was clay 594 g·kg−1, silt 147 g·kg−1 and sand 258 g·kg−1. Soil analysis methods used in this study are described in a soil analysis manual published by EMBRAPA [20] .
Cover crops used for the experiment are listed in Table 1. Zinc levels used were 0, 10, 20 and 40 mg Zn·kg−1 of soil and applied as zinc sulfate (23% Zn). Basic fertilizer rates used were N 200 mg·kg−1, P 200 mg·kg−1 and K 200 mg·kg−1. Nitrogen was applied as urea, P applied as triple superphosphate and K applied as potassium chloride. One g of dolomitic lime (1 g·kg−1 soil) was thoroughly mixed with the soil six weeks before sowing of the cover crop seeds. The experiment was conducted in plastic pots with 7 kg soil in each pot. The experimental design was a randomized block arranged in a split plot. Zinc levels were the main plots and cover crops were the sub-plots. Each experimental unit was replicated three times. After germination, four plants were maintained in each pot. Plants were harvested 50 days after sowing. After harvesting the shoots, roots were removed from the soil manually and washed in water and distilled water several times. Maximum root length was measured. Plant materials were dried in a forced draft oven at 70˚C to a constant weight to determine the dry weight of shoots and roots. Plant shoots were ground and analyzed for Zn according to the methods described by Silva [21] .
Zinc use efficiency was calculated by using the following equation:
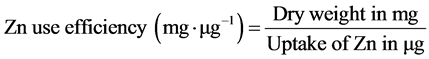
SAS was used for Analysis of Variance and quadratic regression models were determined to describe the shoot dry weight, root dry weight and maximum root length responses to Zn application. Similar quadratic

Table 1. Common and scientific names of ten legume cover crop species used in the experiment.
regression models were used to describe Zn rate versus Zn concentration, Zn uptake and Zn use efficiency in the plants. Similarly, the relationship between soil chemical properties and shoot dry weight was determined by quadratic regression model to calculate values of soil chemical properties for maximum dry weight. The quadratic model is a second order polynomial function written as:

where Y = the estimated yield, X = application rate of Zn. The a, b, c, are coefficients estimated by fitting the model to the data. The quadratic function assumes that cover crop shoot dry weight, root dry weight and maximum root length, Zn concentration and uptake in the plant tissues will increase at a decreasing rate as the Zn application rate increases until the maximum yield is achieved at a determined Zn rate.
3. Results and Discussion
3.1. Shoot Dry Weight
The shoot dry weight of the ten cover crops was significantly influenced by Zn level, cover crop treatments and Zn × cover crops interaction (Table 2). The significant interaction between Zn × cover crops indicates variation in dry weight of shoots of cover crops with the variation in soil Zn rates. At the 0 mg Zn·kg−1 soil rate, shoot dry weight varied from 0.38 g·plant−1 produced by calopo to 7.95 g·plant−1 produced by jack bean, with an average of 2.67 g·plant−1. At 10 mg Zn·kg−1 level, shoot dry weight varied from 0.83 to 9.27 g·plant−1, with an average value of 4.01 g·plant−1. At 20 mg·kg−1 Zn level, dry weight of shoots varied from 0.79 to 9.98 g·plant−1, with an average value of 3.99 g·plant−1. At the maximum Zn level (40 mg·kg−1), shoot dry weight varied from 0.70 to 9.31 g·plant−1 with an average value of 9.31 g·plant−1. However shoot dry weight of all the cover crops decreased at this level of Zn application. Across all four Zn levels, minimum dry weight of 0.73 g·plant−1 was produced by pueraria and maximum dry weight of 9.13 g·plant−1 was produced by jack bean, with an average value of 3.45 g·plant−1. Significant variation in shoot dry weight among cover crops has been reported by Fageria et al. [22] . Low fertility is a major constraint for good growth of legume cover crops in tropical plantation crops [22] . Similarly, Baligar and Fageria [16] reported that growth of cover crops is determined genetically and is affected by environmental variables including fertilizers.
The increase in shoot dry weight fit quadratic curves with increasing Zn levels in the range of 0 to 40 mg·kg−1 (Table 3). The maximum shoot dry weight was obtained with the application of 16 mg Zn·kg−1 soil for showy crotalaria to 32 mg Zn·kg−1 for calopo. The maximum shoot dry weight of the ten cover crops averaged together was obtained with the addition of 22 mg Zn·kg−1. Fageria and Stone [4] reported that Zn deficiency is very common in crops grown on Brazilian Oxisols. Fageria et al. [1] reported that Zn deficiency in highly weathered Oxisols and Ultisols is due to low levels of this element in the parent materials. In addition, Fageria [14] reported that liming is an essential and dominant practice to improve yields of crops on highly weathered acid

Table 2. Influence of soil Zn on shoot dry weight (g·plant−1) of ten tropical legume cover crops.
**Significant at the 1% probability level. Means followed by the same letter in the same column are not statistically significant at the 5% probability level by Tukeys test. Average values were compared in the same line.

Table 3. Relationship between Zn rate and shoot dry weight (SDW) of ten tropical legume cover crops.
**,NSSignificant at the 1% probability level and not significant, respectively. 1ZnRMTDW = Zn rate for maximum tops dry weight in mg·kg−1 soil.
soils. However, liming may induce Zn deficiency due to adsorption of this element on soil colloids by increasing soil pH [23] . Alloway [2] reported that Zn deficiency is by far the most ubiquitous micronutrient deficiency problem in the world. All crops can be affected by Zn deficiency but there is variation in Zn deficiency in crop species and genotypes within species [2] . Figure 1 shows growth of shoots of cover crops at different Zn levels. There was improvement in shoot growth with the addition of Zn and differences were evident among the cover crop species tested.
3.2. Root Growth
Root dry weight was significantly affected by Zn level, cover crop treatment and Zn × cover crop interaction (Table 4). The Zn × cover crop interaction indicates that there was variation in root dry weight among cover crops species with the variation in Zn levels. The root dry weight at the lowest Zn level (0 mg Zn·kg−1) varied from 0.03 to 0.84 g·plant−1, with an average value of 0.22 g·plant−1. At 10 mg Zn·kg−1 level, root dry weight varied from 0.05 to 1.05 g·plant−1, with an average value of 0.36 g·plant−1. Similarly, at 20 mg Zn·kg−1 level, root dry weight varied from 0.03 to 0.93 g·plant−1, with an average value of 0.29 g·plant−1. At the highest Zn level (40 mg Zn·kg−1), root dry weight varied from 0.03 to 0.80 g·plant−1, with an average value of 0.26 g·plant−1.



Figure 1. Growth of selected cover crops at different soil Zn levels.

Table 4. Influence of soil Zn on root dry weight (g·plant−1) of ten tropical legume cover crops.
**Significant at the 1% probability level. Means followed by the same letter in the same column are not statistically significant at the 5% probability level by Tukeys test. Average values were compared in the same line.
Across all four Zn levels, root dry weight varied from 0.05 to 0.90 g·plant−1, with an average value of 0.41 g·plant−1. Black velvet bean produced the highest root dry weight at all four Zn levels. The root weight in diverse crop species is genetically controlled as well as influenced by environmental factors, especially by mineral nutrition [24] . Variation in root weight of cover crop species has been widely reported by Fageria [14] and Fageria and Moreira [25] .
The increase in root dry weight was quadratic when Zn was applied in the range of 0 to 40 mg·kg−1 (Table 5). Among the ten cover crop species, root dry weights of only four (smooth crotalaria, pueraria, lablab and gray velvet bean) increased significantly with increasing soil Zn levels. The rate of soil Zn for maximum root dry weight of these crops was 20 mg Zn·kg−1 for smooth crotalaria, 18 mg Zn·kg−1 for pueraria, 24 mg Zn·kg−1 for lablab and 20 mg Zn·kg−1 for velvet bean. The average Zn level for maximum root dry weight of the ten cover crops was 22 mg Zn·kg−1. Hence, it can be concluded that Zn requirements varied among the cover crop species. Fageria [24] and Fageria and Moreira [25] reported that root weight of crop plants increased in a quadratic fashion with the increasing levels of added Zn.
Maximum root length was significantly affected by Zn and cover crop treatments (Table 6). The Zn × cover crop interaction was also significant for maximum root length. Hence, it can be concluded that variation in maximum root length among cover crop species was not consistent across Zn rates. Maximum root length averaged across the four Zn levels varied from 9.75 for smooth crotalaria to 34 cm for black velvet bean, with an overall average of 24.5 cm. It has been extensively reported that in Brazilian Oxisols, variation in maximum root length of cover crops was observed with the addition of different fertilizer rates [14] [24] . The increase in maximum root length was significant and quadratic for seven of the cover crop species as well as the average of the ten cover crop species (Table 7). The predicted Zn rate for maximum root length varied from 9 mg Zn·kg−1 for smooth crotalaria to 25 mg Zn·kg−1 for lablab, with an average value of 17 mg Zn·kg−1. Similar responses of

Table 5. Relationship between soil Zn rate and root dry weight (RDW) of ten legume cover crops.
*,**Significant at the 5 and 1% probability levels, respectively. 1ZnRMRDW = Zn rate for maximum root dry weight in mg·kg−1 soil.

Table 6. Influence of soil Zn on maximum root length (MRL, cm·plant−1) of ten tropical legume cover crops.
**Significant at the 1% probability level. Means followed by the same letter in the same column are not statistically significant at the 5% probability level by Tukeys test. Average values were compared in the same line.

Table 7. Influence of soil Zn on maximum root length (MRL, cm·plant−1) of ten tropical legume cover crops.
**, NSSignificant at the 1% probability level and not significant, respectively. 1ZnRMLW = Zn rate for maximum root length in mg·kg−1 soil.
root length have been reported in crop plants with the addition of Zn in Brazilian Oxisol [14] [26] . Wang et al. [27] also reported that root growth is extremely sensitive to wide range of soil physical, chemical and biological factors, including mineral nutrition. Figure 2 shows root growth of cover crop species at different Zn levels. There were differences in root growth among cover crop species and also differences with the addition of different levels of Zn fertilizer.
3.3. Zinc Concentration, Uptake and Use Efficiency
Zn concentration (content per unit dry matter), Zn uptake (concentration × dry matter) and Zn use efficiency (dry weight per unit of Zn uptake) were significantly influenced by Zn level and cover crop (Tables 8-10). Overall, Zn concentration increased in a quadratic manner with the addition of Zn to the soil from 0 to 40 mg·kg−1 (Y = 18.23 + 6.14X − 0.09X2, R2 = 0.98**). The Zn concentrations in the cover crop shoots at various soil applied Zn levels were comparable to Zn concentrations reported for other legumes [28] . The Zn concentration of smooth crotalaria was always higher than the others and was two times higher than the average at the 40 mg·kg−1 level.

Figure 2. Root growth of selected cover crops at different soil Zn levels.

Table 8. Influence of soil Zn on Zn concentration (mg·kg−1) in the shoots of ten cover crops.
**Significant at the 1% probability level. Means followed by the same letter in the same column are not statistically significant at the 5% probability level by Tukeys test. Average values were compared in the same line.

Table 9. Influence of soil Zn on Zn uptake (mg·kg−1) in the shoots of ten cover crops.
**Significant at the 1% probability level. Means followed by the same letter in the same column are not statistically significant at the 5% probability level by Tukeys test. Average values were compared in the same line.

Table 10. Influence of soil Zn on Zn use efficiency (mg·µg−1) in the shoots of ten cover crops.
**Significant at the 1% probability level. Means followed by the same letter in the same column are not statistically significant at the 5% probability level by Tukeys test. Average values were compared in the same line.
The average Zn uptake across four Zn levels varied from 41.43 µg·plant−1 to 374 µg·plant−1, with an average of 211 µg·plant−1 (Table 9). Overall, Zn uptake varied from 39.07 µg·plant−1 at the lowest Zn level to 305.68 µg·plant−1 at the 20 mg Zn·kg−1 soil. The uptake of Zn was quadratic and decreased at the highest Zn level (Y = 50.89 + 28.57X − 0.051X2, R2 = 0.98**). The cover crop species showed differential uptake characteristics at given levels of soil Zn. Interspecific variation in Zn uptake has been reported for other legume crops [1] [18] .
Zinc use efficiency varied from 18.25 to 40.80 mg·µg−1, with an average value of 26.14 mg·µg−1 across the four Zn levels (Table 10). The Zn use efficiency decreased with increasing soil Zn levels in a quadratic fashion (Y = 58.58 − 4.36X + 0.08X2, R2 = 0.92**). The decrease in nutrient use efficiency with increasing soil nutrient levels is widely reported in the literature [12] [24] . The decrease in nutrient use efficiency at higher nutrient levels may be related to saturation of the plant’s capacity for nutrient uptake [12] [14] [15] . Cover crops that have high Zn use efficiency might produce more yield when grown on Zn deficient soils. In the current study Jack bean, black velvet bean, pueraria and gray velvet bean had high Zn use efficiency at low and no application of Zn. Interspecific variation in nutrient use efficiency among tropical crops has been reported [16] [18] [19] .
4. Conclusion
Cover crops are important components of cropping systems in maintaining sustainable crop production and soil quality. Results of this study show that Zn fertilization increased cover crop shoot dry weight, root dry weight and maximum root length; however response to applied Zn varied from species to species. Similarly, Zn concentration (content per unit dry weight), Zn uptake (concentration × dry weight) and Zn use efficiency also varied from crop species to crop species. Hence, selection of suitable cover crops for Zn use efficiency is an important strategy in successful production of cover crops on Zn deficient soils. Overall, applied Zn rate needed for maximum dry weight of shoots and roots is about 22 mg Zn·kg−1 soil when the original level of the native soil is about 0.2 mg Zn·kg−1 determined by Mehlich 1 method. Among the ten cover crop species, jack bean, black velvet bean and lablab produced highest dry weight of shoots as well as roots. The cover crops which produced minimum dry weight of shoots and roots were calopo, pueraria, and smooth crotalaria. Jack bean, black velvet bean, and gray velvet bean had high Zn use efficiency at low soil Zn level and hence these cover crops might be suitable cover crops for soils with low available Zn.
Acknowledgements
We thank L. F. Stone for his excellent review and suggestions on the manuscript.