1. Introduction
Atmospheric aerosol particles reveal changes in their microphysical and optical characteristics with their am- bient relative humidity (RH) due to the water uptake [1] - [3] . Since atmospheric aerosols are far from being a single component, the question is how relative humidity influences the optical properties of natural aerosol mix- tures, which can contain both soluble and insoluble components. These hygroscopic atmospheric aerosols un- dergo droplet growth, evaporation and phase transformation from a solid particle to a saline droplet which usually occurs spontaneously when the RH reaches a level called the deliquescence humidity. Its value is spe- cific to the chemical composition of the aerosol particle [4] [5] . Ambient aerosols generally composed of exter- nal and internal mixtures of particles with different chemical compounds, chemical compositions and physical properties such as soot, sulphate, nitrate, organic carbon and mineral dust. The state of mixing of these compo- nents is crucial for understanding the role of aerosol particles in the atmosphere.
The hygroscopicity of these aerosol particles plays an important role in the atmosphere, because they influence the radiative budget of the earth directly by scattering and absorbing the incoming sunlight and indirectly by serving as cloud condensation nuclei for the formation of fog and cloud droplets [6] . Depending on the chemical composition, aerosol particles can take up large amounts of water compared to their dry state as relative humid- ity increases to 100% and thus radically increase their size and change their optical properties [7] . The aerosol hygroscopic properties are very crucial for the understanding of the aerosol effects on climate via their effects on clouds, since the hygroscopic growth measured at sub saturation is closely related to the ability of aerosol par- ticles to activate and form cloud droplets [8] [9] . Changes in particle size and phase with relative humidity modify heterogeneous atmospheric chemistry, cloud and fog formation processes, and visibility. As climate models im- prove, they must incorporate the dependence of aerosol radiative effects on RH. Because of the important role that water vapor plays in determining aerosol effects, it is critical to develop the understanding that is required to reliably represent water vapor-particle interactions in models.
The Kohler Equation is often used to describe both the hygroscopic growth and the activation of aerosol par- ticles to cloud droplets, based on the aerosol’s physicochemical properties [10] . However, these detailed proper- ties are not always available for ambient aerosols. Size-dependent mixing states of various chemical composi- tions also increase the complexity. Recently, several single-parameter schemes have been proposed to simplify the Kohler Equation. Hygroscopicity parameters such as κ and pion have been defined as proxies of chemical composition to represent aerosol hygroscopic growth as well as the ability of aerosol particles to become cloud condensation nuclei (CCN) [11] [12] . Moreover, Rissler et al. [9] recently overviewed several models which describe the aerosol hygroscopicity and the CCN activation.
Hygroscopic properties of aerosol particles were investigated in marine environments in several field studies in the last ten years [13] - [15] . Based on a review of observational data, Andreae and Rosenfeld [16] suggested that continental and marine aerosols on average tend to cluster into relatively narrow ranges of effective hy- groscopicity (continental κ = 0.3 ± 0.1; marine κ = 0.7 ± 0.2). Recent field studies are largely consistent with this view, but they show also systematic deviations for certain regions and conditions. For example, Gunthe et al. [17] reported a characteristic value of κ = 0.15 for pristine tropical rainforest aerosols in central Amazonia, which are largely composed on secondary organic matter.
Sea salt aerosols are also of interest since they are the dominant kind by mass over the oceans [18] [19] .
The main parameter used to characterize the hygroscopicity of the aerosol particles is the aerosol hygroscopic growth factor GF(RH), which is defined as the ratio of the particle diameter at any RH to the particle diameter at RH = 0% [8] [20] .
Changes in the aerosol optical properties resulting from the particle hygroscopic growth are described by en- hancement factors f (RH), which, for each optical parameter χ, are defined as the ratio between its values deter- mined in any conditions χ (RH) and those determined in dry conditions χ (RH = 0%). Technically, the enhance- ment factor for scattering and hemispherical backscattering can be determined for a chosen RH by using two nephelometers performing measurements at the chosen RH and in dry conditions (RH = 0%), respectively [21] - [26]
The aim of this study is to determine the aerosols hygroscopic growth and enhancement factors for maritime tropical aerosols from the data extracted from OPAC. One and two variables parameterizations models will be performed to determine the relationship of the particles’ hygroscopic growth and enhancement parameters with the RH. Angstrom coefficients are used to determine the particles’ type and the type mode size distributions.
2. Methodology
The models extracted from OPAC are given in Table 1.
Where water soluble components consists of scattering aerosols, that are hygroscopic in nature, such as sul- fates and nitrates present in anthropogenic pollution, while sea-salt accumulation and coarse modes are two kinds of salt contained in seawater that are more hygroscopic than water soluble.
The software package OPAC is written in FORTAN programming language. It easily provides optical proper- ties in the solar and terrestrial spectral range of atmospheric particulate matter. Microphysical and optical prop- erties of ten (10) aerosol components, which are considered as typical cases, are stored as ASCII files. The opti- cal properties of the aerosols are calculated on the basis of the microphysical data (size distribution and spectral refractive index) under the assumption of spherical particles. Data are given for up to 61 wavelengths between 0.25 and 40 µm and up to eight values of the relative humidity.
The globally averaged direct aerosol Radiative forcing, ∆FR, for absorbing aerosols was calculated using the equation derived by Chylek and Wong [28] as:
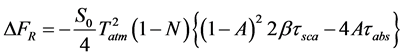 (1)
(1)
where S0 is a solar constant, Tatm is the transmittance of the atmosphere above the aerosol layer, N is the fraction of the sky covered by clouds, A is the albedo of underlying surface, β is the upscattering fraction of radiation scattered by aerosol into the atmosphere while τsca and τabs are the aerosol layer scattering and absorptions opti- cal thickness respectively. The above expression gives the radiative forcing due to the change of reflectance of the earth-aerosol system. The upscattering fraction is calculated using an approximate relation [29]
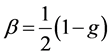 (2)
(2)
where g is the asymmetric parameter. The global averaged albedo A = 0.22 over land and A = 0.06 over the ocean with 80% of aerosols being over the land; solar constant of 1370 Wm−2, the atmospheric transmittance is taken to be Tatm = 0.79 [30] and cloudness N = 0.6.
The aerosol’s hygroscopic growth factor gf(RH), [8] [31] is defined as:
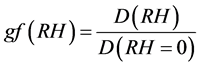 (3)
(3)
where RH is taken for seven values 50%, 70%, 80%, 90%, 95%, 98% and 99%.
But since atmospheric aerosols consist of more and less hygroscopic sub fractions so the information on the hygroscopicity modes was merged into an “over-all” or “bulk” hygroscopic growth factor of the mixture, gfmix(RH), representative for the entire particle population as:
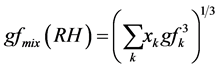 (4)
(4)
The effective or volume equivalent radius of the mixture was determined using the relation
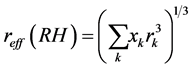 (5)
(5)
![]()
Table 1. Compositions of aerosols types at 0% RH [27] .
where the summation is performed over all compounds present in the particles and xk represent their respective volume fractions, using the Zdanovskii-Stokes-Robinson relation [32] - [35] . Solute-solute interactions are neg- lected in this model and volume additivity is also assumed. The model assumes spherical particles, ideal mixing (i.e. no volume change upon mixing) and independent water uptake of the organic and inorganic components.
Equations (4) and (5) can also be computed using the xk as the corresponding number fractions [36] [37] .
We now proposed the xk to represent the mass mix ratio of the individual particles.
The RH dependence of gfmix(RH) can be parameterized in a good approximation by a one-parameter equation, proposed e.g. by Petters and Kreidenweis [11] as:
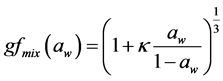 (6)
(6)
Here, aw is the water activity, which can be replaced by the relative humidity RH, if the Kelvin effect is neg- ligible, as for particles with sizes more relevant for light scattering and absorption. The coefficient κ is a simple measure of the particle’s hygroscopicity and captures all solute properties (Raoult effect), that is, it is for the en- semble of the particle which can be defined in terms of the sum of its components. In an ensemble of aerosol particles, the hygroscopicity of each particle can be described by an “effective” hygroscopicity parameter κ [11] [38] . Here “effective” means that the parameter accounts not only for the reduction of water activity by the so- lute but also for surface tension effects [17] [39] [40] . It also scales the volume of water associated with a unit volume of dry particle [11] and depends on the molar volume and the activity coefficients of the dissolved compounds [41] . The κ value derived a particle of a given composition may vary, depending upon the size molar mass, the activity and RH it is derived at.
For atmospheric aerosols, the range of κ typically varies from as low as ∼0.01 for some combustion aerosol particles up to ∼1 for sea-salt particles [11] [16] [42] [43] .
The following sub-divisions at 85% RH were made by Swietlicki et al., [8] and Liu et al., [44] ; as nearly-hydro- phobic particles (NH): k <= 0.10 (gfmix <= 1.21), less-hygroscopic particles (LH): k = 0.10 − 0.20 (gfmix = 1.21 − 1.37); more-hygroscopic particles (MH): k > 0.20 (gfmix > 1.37).
Making κ as the subject of Equation (6), we get
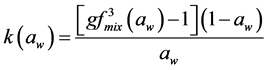 (7)
(7)
Humidograms of the ambient aerosols obtained in various atmospheric conditions showed that gfmix(RH) could as well be fitted well with a γ-law [14] [45] - [48] as;
 (8)
(8)
Making g as the subject of Equation (8) we get
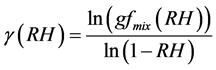 (9)
(9)
The bulk hygroscopicity factor B under subsaturation RH conditions was determined using the relation:
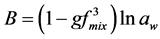 (10)
(10)
where aw is the water activity, which can be replaced by the RH as explained before.
The impact of hygroscopic growth on the optical properties of aerosols is usually described by the enhance- ment factor :
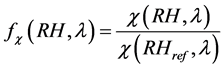 (11)
(11)
where in our study RHref was 0%, and RH was taken for seven values of 50%, 70%, 80%, 90%, 95%, 98% and 99%.
In general, the relationship between 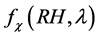 and RH is nonlinear [49] . In this paper, we determine the empirical relations between the enhancement parameter and RH [50] as:
and RH is nonlinear [49] . In this paper, we determine the empirical relations between the enhancement parameter and RH [50] as:
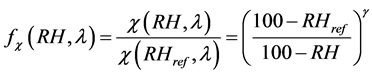 (12)
(12)
The g known as the humidification factor represents the dependence of aerosol optical properties on RH, which results from the changes in the particles sizes and refractive indices upon humidification. The use of g has the advantage of describing the hygroscopic behavior of aerosols in a linear manner over a broad range of RH values; it also implies that particles are deliquesced [51] , a reasonable assumption for this data set due to the high ambient relative humidity during the field study. The g parameter is dimensionless, and it increases with increasing particle water uptake.
Making g as the subject of Equation (12) and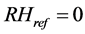 , we get
, we get
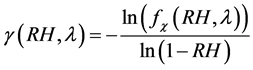 (13)
(13)
From previous studies, typical values of γ for ambient aerosol ranged between 0.1 and 1.5 [51] - [53] .
Two parameters empirical relation was also used [49] [54] as;
![]() (14)
(14)
Equations (12) and (14) are verified at wavelengths 0.25, 0.45, 0.55, 0.70, 1.25, and 2.50 µm.
To determine the effect of particles distributions as a result of change in RH, the Angstrom exponent was de- termined using the spectral behavior of the aerosol optical depth, with the wavelength of light (λ) was expressed as inverse power law [55] :
![]() (15)
(15)
The Angstrom exponent was obtained as a coefficient of the following regression,
![]() (16)
(16)
However, Equation (16) was determined as non-linear (that is the Angstrom exponent itself varies with wave- length), and a more precise empirical relationship between the optical depth and wavelength was obtained with a 2nd-order polynomial [56] - [66] as:
![]() (17)
(17)
and then we proposed the cubic relation to determine the type of mode distribution as:
![]() (18)
(18)
where β, α, α1, α2, α3 are constants that were determined using regression analysis with SPSS16.0. forwondows.
We also determined the exponential dependence of the aerosol optical thickness on relative humidity as done by Jeong et al. [49] as;
![]() (19)
(19)
where A and B are constants determined using regression analysis with SPSS 16.0 and was computed at wave- lengths 0.25, 1.25 and 2.50 mm.
We finally determine the effect of hygroscopic growth on the effective refractive indices of the mixed aero- sols using the following formula [67] :
![]() (20)
(20)
The relation between dielectrics and refractive indices is
![]() (21)
(21)
We also used another mixing rule formula that has been used in the several widely employed databases of aerosol optical properties [27] [68] - [71] as:
![]() (22)
(22)
where fi and εi are the volume fraction and dielectric constant of the ith component and ε0 is the dielectric con- stant of the host material. For the case of Lorentz-Lorentz [72] [73] , the host material is taken to be vacuum, ε0 = 1.
We then proposed the fi to be mass mix ratios and number mix ratios, to determine the advantage of one over the other.
The computations of Equations (20), (21) and (22) were done using the complex functions of Microsoft Excel 2010.
3. Results and Discussions
3.1. Radiative Forcing
From Figure 1, at 0% RH the RF is almost constant with a value of −5 Wm−2 with respect to wavelengths. As the RH increases, the RF(cooling) increases with the increase in RH, but become non-linear with respect to wave- lengths. At higher RHs the increase in RF(cooling) is more at larger wavelengths than shorter wavelengths. This shows the dominance of coarse mode particles.
3.2 Microphysical Properties
From Table 2, it can be observed that there are increases in gfmix, reff and g, while there are decreases in B and k with the increase in RH.
The results of the parameterizations by one parameter of Equations (6) and (8) using number mix ratios are:
k = 0.1844, R2 = 0.9549 from Equation (6);
𝛾 = −0.2146, R2 = 0.9964 from Equation (8).
From the observations of R2, it can be seen that the data fitted the Equations very well.
From Table 3, it can be observe that there are increases in gfmix, reff and g, while there are decreases in B and k with the increase in RH.
![]()
Figure 1. A graph of radiative forcing against wavelengths.
![]()
Table 2. The table of hygroscopic growth factors, effective radii, B (bulk hygroscopicity), k (hygroscopicity) and g (Humidi- fication factor), of the aerosols using number mix ratio.
![]()
Table 3. The table of hygroscopic growth factors, effective radii, B (bulk hygroscopicity), k (hygroscopicity) and g (Humidi- fication factor), of the aerosols using volume mix ratio.
The results of the parameterizations by one parameter of Equations (6) and (8) using volume mix ratio are:
k = 1.0740, R2 = 0.9980 from Equation (6);
𝛾 = −0.3550, R2 = 0.9866 from Equation (8).
From the observations of R2, it can be seen that the data fitted the equations very well.
From Table 4, it can be observe that there are increases in gfmix, reff and g, while there are decreases in B and k with the increase in RH.
The results of the parameterizations by one parameter of Equations (6) and (8) using mass mix ratio are:
k = 1.0731, R2 = 0.9980 from Equation (6);
𝛾 = −0.3548, R2 = 0.9867 from Equation (8).
From the observations of R2, it can be seen that the data fitted the equations very well.
From Figure 2, it can be observed that the plots of gfmix using mass and volume mix ratios are the same and higher than the gfmix using number mix ratios. It can also be observed that all the plots satisfy power law.
From Figure 3, it can be observed that the plots of reff using mass and volume mix ratios are the same and higher than the reff using number mix ratios. It can also be observed that all the plots satisfy power law.
From Figure 4, it can be seen that B decreases with the increase in RH in a nonlinear form. It can also be ob- serve that number mix ratio has the least while volume and mass mix ratios higher and the same. They also sa- tisfy power law.
From Figure 5, it can be seen that k decreases with the increase in RH in almost power law form. It can also be observe that number values mix ratio has the least while volume and mass mix ratios have higher values and the same.
From Figure 6, it can be seen that the plots of g increase with the increase in RH in almost non-linear form, though they can satisfy power law. The plots using mass and volume mix ratios are higher in magnitude com- pared to number mix ratio.
3.3. Optical Properties
From Figure 2, as from 0% to 90% RH, the optical depth decreases monotonically with wavelengths and in- creases with the increase in the RH as a result of the hygroscopic growth. But as the RH increases from 95% to 99% RH the optical depth the monotonical behavior continue to decrease with respect to wavelength and RH. This behavior may be attributed to the dominance of coarse particles, which increases with the increase in
![]()
Table 4. The table of hygroscopic growth factors, effective radii, B (bulk hygroscopicity), k (hygroscopicity) and g (Humidi- fication factor), of the aerosols using mass mix ratio.
![]()
Figure 2. A graph of gfmix against RH using number, volume and mass mix ratios using the data from Tables 2-4.
![]()
Figure 3. A graph of effective radii against RH using number, volume and mass mix ratios using the data from Tables 2-4.
![]()
Figure 4. A graph of B (bulk hygroscopicity) against RH using number, volume and mass mix ratiosusing the data from Tables 2-4.
![]()
Figure 5. A graph of k (hygroscopicity) against RH using number, volume and mass mix ratios using the data from Tables 2-4.
hygroscopic growth. The behavior of the power law decreases with the increase in RHs, this also signifies the increase in the dominance of coarse particles with the increase in RH.
Using the data for plotting Figure 7, the results of exponential relation between optical depth and RHs using Equation (19) are:
At λ = 0.25 μ, A = 0.0412, B = 1.2079, R2 = 0.6283
At λ = 1.25 μ, A = 0.0093, B = 2.3547, R2 = 0.7783
At λ = 2.50 μ, A = 0.0029, B = 3.2068, R2 = 0.7800
The relation between optical depth and RH shows increase in R2 and exponent B with the increase in wave- length, and this signifies the dominance of coarse particles.
![]()
Figure 6. A graph of g against RH using number, volume and mass mix ratios.
![]() Wavelengths (μm)
Wavelengths (μm)
Figure 7. A graph of optical depth against wavelengths.
From Table 5, at 0% RH, the value of a is greater than 1, and this signifies the dominance of fine particles. But as the RH increases, it continues to decrease signifying the increase in the dominance of coarse particles. From the quadratic part, the negative curvature (a2) decreases with the increase in RH and became positive and increased further and also the values of R2 decreases with the increase in RH, and these signify increase in the concentration of coarse particles with the increase in RH. The increase in the coarse particle size can be seen in Tables 2-4 where it can be seen that the effective radii increase with the increase in RHs. The cubic part signifies mode distributions as bi-modal with the dominance of coarse particles, because of the decrease in a1.
Figure 8 shows that the enhancement factors increase with the increase in RH and wavelengths in almost power law form. The nature of the increase with the increase in RH and wavelengths reflects the coarse and highly hygroscopic properties of the aerosols.
The results of the fitted curves of Equations (12) and (14) using the data for plotting Figure 8 are presented as follows;
![]()
Table 5. The results of the Angstrom coefficients for optical depth using Equations (16), (17) and (18) at the respective rela- tive humidities using regression analysis with SPSS16 for windows.
![]() Wavelength (μm)
Wavelength (μm)
Figure 8. A graph of enhancement parameter for optical depth against wave- lengths.
For a single parameter using Equation (12).
At λ = 0.25 μ, γ = 0.3069, R2 = 0.9932
At λ = 0.45 μ, γ = 0.3783, R2 = 0.9965
At λ = 0.55 μ, γ = 0.4153, R2 = 0.9970
At λ = 0.70 μ, γ = 0.4643, R2 = 0.9969
At λ = 1.25 μ, γ = 0.6233, R2 = 0.9921
At λ = 2.50 μ, γ = 0.8462, R2 = 0.9925
For two parameters using Equation (14).
At λ = 0.25 μ, a = 0.9326, b = −0.3287, R2 = 0.9796
At λ = 0.45 μ, a = 0.9900, b = −0.3815, R2 = 0.9848
At λ = 0.55 μ, a = 1.0298, b = −0.4061, R2 = 0.9869
At λ = 0.70 μ, a = 1.1075, b = −0.4323, R2 = 0.9911
At λ = 1.25 μ, a = 1.3781, b = −0.5228, R2 = 0.9975
At λ = 2.50 μ, a = 1.5364, b = −0.7117, R2 = 0.9985
For both one and two parameters, the values of R2 signify excellent relation and the increase in the expo- nents signifies increase in relation with wavelength. This signifies the dominance of coarse particles.
Figure 9 shows increase in g with the increase in RHs and wavelengths, and the increase is more significant at higher RHs and wavelengths. This also shows the dominance of coarse and very hygroscopic particles.
Comparing Figure 7 and Figure 10, it can be seen that they are similar, except that the plots in Figure 7 have higher values.
Using the data for plotting Figure 10, the results of exponential relation between extinction coefficient and RHs using Equation (19) are:
At λ = 0.25, A = 0.0110, B = 2.3400, R2 = 0.7856
![]() Wavelength (μm)
Wavelength (μm)
Figure 9. A graph of g against wavelength using Equation (13).
![]() Wavelength (μm)
Wavelength (μm)
Figure 10. A graph of extinction coefficients against wavelengths.
At λ = 1.25 μ, A = 0.0069, B = 2.7675, R2 = 0.8272
At λ = 2.50 μ, A = 0.0028, B = 3.3947, R2 = 0.8007
The relation between optical depth and RH shows increase in R2 and exponent B with the increase in wave- length, and this signifies the dominance of coarse particles.
At Table 6, from the linear part with the values of a is less than 1 and together with the values of R2 continue decreasing with the increase in RH signify the dominance of coarse particles. From the quadratic part, the nega- tive curvature (a2) decreases with the increase in RH and became positive and increase further and this also sig- nifies increase in the concentration of coarse particles as a result of coagulation, aging and sedimentation of fine particles with the increase in RH. This increase can be seen in Tables 2-4 where it can be observe that the effec- tive radii increase with the increase in RHs. The cubic part signifies mode distributions as bi-modal with the dominance of coarse mode particles.
From Figure 11, comparing Figure 8 and Figure 11, it can be observe that they are similar, except that the values of the plots for Figure 11 are higher than those in Figure 8.
![]()
Table 6. The results of the Angstrom coefficients for extinction coefficient using Equations (16), (17) and (18) at the respec- tive relative humidities using regression analysis with SPSS16 for windows.
![]() Wavelength (μm)
Wavelength (μm)
Figure 11. A graph of enhancement parameter for extinction coefficients against wavelengths.
The results of the fitted curves of Equations (12) and (14) using the data for plotting Figure 11 are presented as follows;
For a single parameter using Equation (12).
At λ = 0.25 μ, γ = 0.6223, R2 = 0.9907
At λ = 0.45 μ, γ = 0.6429, R2 = 0.9888
At λ = 0.55 μ, γ = 0.6507, R2 = 0.9883
At λ = 0.70 μ, γ = 0.6651, R2 = 0.9865
At λ = 1.25 μ, γ = 0.7461, R2 = 0.9833
At λ = 2.50 μ, γ = 0.9040, R2 = 0.9891
For two parameters using Equation (14).
At λ = 0.25 μ, a = 1.4203, b = −0.5124, R2 = 0.9976
At λ = 0.45 μ, a = 1.4879, b = −0.5184, R2 = 0.9970
At λ = 0.55 μ, a = 1.5093, b = −0.5217, R2 = 0.9969
At λ = 0.70 μ, a = 1.5770, b = −0.5225, R2 = 0.9975
At λ = 1.25 μ, a = 1.7773, b = −0.5660, R2 = 0.9992
At λ = 2.50 μ, a = 1.7491, b = −0.7289, R2 = 0.9988
For both one and two parameters, the values of R2 signify excellent relation and the increase in the exponents signifies increase in relation with wavelength. This signifies the dominance of coarse particles.
From Figure 12, comparing Figure 9 and Figure 12, it can be seen that they are the similar, except that the values of the plots in Figure 12 are higher.
From Figure 13, comparing Figure 7, Figure 10 and Figure 13, it can be seen that they are similar.
From Figure 14, comparing Figure 8, Figure 11 and Figure 14, it can be observe that they are similar.
Figure 15 shows that absorption is very small at shorter wavelengths, but as from 1.25 µm the absorption in- creases with both wavelengths and RH. This shows that the increase in the absorption is more at larger particles and higher wavelengths as the RH increases and this shows that hygroscopic growth has more effect on larger particles. It also shows that power law is not obeyed.
Figure 16 shows that it is almost 1 as from 0.25 µm to 1.25 µm and is independent of RH and wavelengths, but as from 1.25 µm it increases with the increase in wavelength and RHs in a non-linear form. This shows the dominance of coarse particles.
Figure 17 shows that hygroscopic growth has caused increase in scattering in the forward direction especially at shorter wavelength and longer wavelengths. Its relation with wavelengths and RH is non-linear. This can be
![]() Wavelength (μm)
Wavelength (μm)
Figure 12. A graph of g against wavelengths using Equation (13).
![]() Wavelength (μm)
Wavelength (μm)
Figure 13. A graph of scattering coefficients against wavelengths.
![]() Wavelength (μm)
Wavelength (μm)
Figure 14. A graph of scattering enhancement against wavelengths.
attributed to high hygroscopicity of these aerosols particles and probably due to internal mixing.
From Figure 18, it can be observe that the plots are non-linear between single scattering albedo with RH and wavelengths. It also shows that as the RH increases, scattering became more dominant at shorter wavelengths while at larger wavelengths, absorptions increases with wavelengths.
3.4 Effective Refractive Indices
Figure 19, the non-liner relationship between the real effective refractive indices and wavelengths at 0% RH signifies the dominance of coarse particles. But as the RH increases, its behaviors with respect to wavelengths and RHs show that the mixtures are internally mixed, probably, because all the particles are very hygroscopic.
![]() Wavelength (μm)
Wavelength (μm)
Figure 15. A graph of absorption coefficients against wavelengths.
![]() Wavelength (μm)
Wavelength (μm)
Figure 16. A graph of absorption enhancement against wavelengths.
Figure 20 shows that increase in RH causes decrease in the effective imaginary refractive indices and as the RH increases, its behaviors with respect to RH and wavelengths show that the mixtures are internally mixed, and this maybe because all the aerosols are all very hygroscopic.
From Figure 21, comparing Figure 19 with Figure 21, it can be seen that they are similar.
From Figure 22, comparing Figure 21 with Figure 22, it can be seen that they are similar.
4. Conclusions
Comparing the three types of gfmix obtained, it can be seen that using volume and mass mix ratios gave better re- presentations of the mixture. These also imply that optical effects of atmospheric aerosols are also more
![]() Wavelength (μm)
Wavelength (μm)
Figure 17. A graph of asymmetric parameter against wavelengths.
![]() Wavelength (μm)
Wavelength (μm)
Figure 18. A graph of single scattering albedo against wavelengths.
closely related to their volume than their number [74] [75] . From the gfmix(RH) observed, it is assumed that the high number fraction of water soluble and sea salt accumulation mode is responsible for its high value. The modeling shows that increase in RH causes decrease in the effective radii, and this is what caused the optical depth and extinction, and scattering coefficients to have higher values at smaller wavelengths with the increase in RH [76] . The relations of these optical and microphysical properties with RH are such that at the deliques- cence point (95% to 99%) this growth with higher humidities increases substantially, making this process strongly nonlinear with relative humidity [76] - [78] .
This shows that the effective hygroscopic growth in smaller particles reveals an immense potential of light scattering enhancement in the forward scattering [79] while in larger particles it causes increase in the backward scattering at high humidities and the potential for being highly effective cloud condensation nuclei. It also shows
![]() Wavelengths (μm)
Wavelengths (μm)
Figure 19. A plot of real effective refractive indices against wavelength using Equation (20).
![]() Wavelengths (μm)
Wavelengths (μm)
Figure 20. A plot of imaginary effective refractive indices against wavelength using Equation (20).
that the mixture is internally mixed for coarse particles because of the nature of the increase in scattering as a result of the hygroscopic growth [67] and the increase in absorption despite decrease in effective imaginary re- fractive indices. Therefore, the behavior of internal mixing and the relative importance of the humidity depen- dences of particle size and index of refraction on the aerosol scattering coefficient for a given substance depend on RH and on the sizes of the particles that provide the dominant contribution to the scattering.
Despite the excellent relation shown for k and g using Equations (6) and (8), but by observing their values us- ing Equations (7) and (9) in Tables 2-4 , it can be concluded that the values of these parameters in Equations (6) and (8) could seriously underestimate those of Equations (7) and (8) most especially at lower RHs. Therefore,
![]() Wavelengths (μm)
Wavelengths (μm)
Figure 21. A plot of real effective refractive indices against wavelength using Equation (22).
![]() Wavelengths (μm)
Wavelengths (μm)
Figure 22. A plot of imaginary effective refractive indices against wavelength using Equation (22).
based on these observations and the observations of hygroscopic parameters in Tables 2-4, it can be concluded that the effective hygroscopic parameters are always dependent on RH. The modeling of gfmix with Equations (6) and (8) shows excellent relation because of the values of R2, and all converge to 1 as the RH or aw approaches 0. The values of R2 for Equation (6) are always less than that of Equation (8), and this may be attributed to the kel- vin effect of Equation (6) which was neglected, and this shows the influence of Kelvin effect.
From the modeling of the enhancement parameters using Equations (12) and (14), it can be observe that there is a very excellent relation. However, based on convergence the convergence behavior of the two models as RH approaches 0, it can be seen that Equation (12) is better, because at this limit it approaches 1, which is what it is supposed to be.
Jeong et al. [49] demonstrated an exponential dependence of the aerosol optical thickness on relative humidi- ty. The behavior of exponential relation between optical depth and extinction coefficients with RH, shows that it is sensitive to the change in the effective radii. This is because it shows that for smaller particles, the relation is better at shorter wavelengths.
The decrease in the Angstrom coefficients in a non-linear form and the decrease in curvature with RH are in line with the increase in the effective radii with the increase in RH. As a consequence of such a non-uniform in- crease, the Angstrom coefficient also becomes a function of RH. The observed variations in Angstrom coeffi- cients can be explained by changes in the effective radii of the mixture resulting from changes in RH: the small- er the number of small aerosol particles, the larger the effective radius and the smaller the Angstrom coefficient. The hygroscopic growth behaviors and the coarse nature of the marine tropical aerosols reveal an immense po- tential of light scattering enhancement at longer wavelength and at high humidities and the potential for being highly effective cloud condensation nuclei.
Finally, the data fitted our models very well and can be used to extrapolate the hygroscopic growth at any RH and enhancement parameters at any RH and wavelengths. The importance of determining gfmix(RH) as a function of RH and volume fractions, mass fractions and number fractions, and enhancement parameters as a function of RH and wavelengths can be potentially important because it can be used for efficiently representing aerosols- water interactions in global models.
The refractive index is highly variable depending on the chemical compositions of aerosols and the type of the mixing state [71] . About the two formulas used for the computations of the effective refractive indices, it can be concluded that they are the same, because they gave almost similar plots at the same computational platform.