A Novel Spectrophotometric Method for Determination of Gabapentin in Pharmaceutical Formulations Using 2,5-Dihydroxybenzaldehyde ()
1. Introduction
Gabapentin (GBP) (1-(aminomethyl) cyclohexane acetic acid) is a new antiepileptic drug which is a structural analogue of neurotransmitter γ-aminobutyric acid (GABA). GBP, unlike GABA, has a cyclohexane molecule system and is able to penetrate through blood-brain barrier. GBP is used for the treatment of partial onset seizures with or without secondary generalized tonic-clonic convulsions in clinical practice [1] [2] . The reported analytical methods for GBP determination include high performance liquid chromatography (HPLC) [3] -[14] , spectrofluorometry [15] [16] , gas chromatography [17] - [19] . Despite that the spectrophotometric techniques had been commonly applied for the determination of some other drugs [20] , only few spectrophotometric methods were reported in literature for determination of GBP, based on the reaction of the primary amino group of GBP with ninhydrine reagent in organic solvent medium [21] - [23] . And other spectrophotometric methods involve the determination of GBP via formation of charge-transfer complexes with π-acceptors in pharmaceutical formulations [24] - [26] .
There is no spectrophotometric method till now for determination of gabapentin in pharmaceutical formulations through formation of Schiff base with DHBA. Our present study was simple, accurate and sensitive spectrophotometric procedure for determination of GBP in pharmaceutical formulations. The method was based on the reaction of primary amino group of GBP drug with DHBA at elevated temperature to produce Schiff base. No interference was observed in the assay of GBP from common excipients. The reaction conditions and application of the method for determination of GBP in pharmaceutical formulation have been established. In addition, the stoichiometric ratio of reactants, the equilibrium constant (Kc) and free energy change (ΔG) were determined.
2. Experimental Work
2.1. Materials
All solutions were prepared from analytical grade materials with pure ethanol 99.8% (Riedel-de Haen Germany). Gabapentin (GBP) pure was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). 2,5-dihydroxybenzal- dehyde (DHBA) reagent was obtained from Alfa Aesor GmbH & Co KG (Zeppelinstra Be Karl Sruche, Germany). Pharmaceutical formulations of GBP such as Gabtin capsules-100 mg (Al-Debeiky pharmaceutical products for Delta pharma, Egypt), and Conventin capsules 100 mg (EVA PHARMA-Egypt) were purchased from local pharmacy.
A stock solution of 5.8 × 10−3 M GBP was prepared by dissolving the accurately weighed amount of the pure GBP in ethanol. Dilute solutions were obtained by accurate dilution. A 5.8 × 10−3 M solution of DHBA was prepared by dissolving the required amount of the reagent (DHBA) in ethanol.
2.2. Instrumentation
An evolution 300 UV-Vis. Spectrophotometer with 1.0 cm matched cells fitted with vision pro software of Thermo Election corporation (Cambridge, U.K.) was used for electronic spectral measurements. To obtain pH readings throughout the experimentation, a microprocessor pH meter (HANNA HI 211) was used.
2.3. General Procedure and Construction of Calibration Graphs
Different aliquots of GBP solution were transferred into test tubes. To each test tube 3 ml of DHBA (2 × 10−3 M) reagent in ethanol and 2 ml ethanol were added, then test tubes were heated on a water-bath at 75 ± 0.15˚C for 15 min. These solutions were transferred to volumetric flasks after cooling and the volume was made up to the mark with ethanol to provide final concentration range of 2 - 50 µg∙ml−1.
The absorbance of the solution was measured against a reagent blank at 445 nm. The calibration graph was prepared by plotting absorbance versus concentration of gabapentin.
2.4. Analysis of Pharmaceutical Formulations
Twenty capsules of each formulation were accurately weighed and powdered in glass mortar. The quantity of the powder equivalent 10 mg of gabapentin was dissolved in 100 ml ethyl alcohol.
The procedure was continued as described in the general procedure. The amount of GBP present in capsule sample solution was determined by fitting the responses into the regression equation.
2.5. Interference from Excipients
Samples were prepared by mixing 10 mg of gabapentin with various amounts of common excipients such as cellulose, lactose, glucose, vitamin C, starch and purified talc. The procedure was continued as mentioned above in the general procedure.
3. Results and Discution
3.1. Spectral Characteristics
Gabapentin (GBP) showed weak absorption band in UV range [21] . UV-spectrophotometric methods is not enough for the determination of GBP in pharmaceutical formulations. Attachment of chromophoric group to GBP increases the sensitivity of its detection. For this reason, DHBA was chosen as a chromagenic reagent. Ninhydrin reagent was used for the determination of an aliphatic primary amine [27] - [29] .
The reaction is usually carried out by heating for a short time in an organic solvent and the reaction product is measured in the visible region depending on the reaction conditions [30] .
3.2. Reaction Mechanism
Shiff bases derived from the condensation of aromatic aldehyde derivatives and aromatic primary amine were synthesized and characterized [31] .
The reaction of GBP with DHBA was studied in ethanol media in the temperature range 45˚C - 80˚C. The visible absorption spectra of solutions were recorded in presence of excess reagent and spectra reflect the formation of yellow product with λmax = 445 nm at T = 75 ± 0.15˚C. Gabapentin interacts with DHBA reagent in ethanol medium at elevated temperature via oxidative deamination of the primary amino group followed by the condensation of the reduced DHBA to form the yellow colored Schiff base as represented by the Scheme 1.
The absorption spectra of gabapentin, DHBA reagent and their reaction product are shown in Figure 1.
3.3. Optimization of Reaction Conditions
The reaction conditions were optimized. The number of parameters such as temperature, time, reagent concentration and solvent were investigated. The optimum conditions were established by changing one variable and observing its effect on the absorbance of the colored product.
3.4. Effect of Solvents
Different solvents such as water, ethanol, methanol, isopropanol, dioxane and acetonitrile have been tested, but the best results were obtained with ethanol.
3.5. The Effect of Concentration of DHBA
The effect of the volume of DHBA (80.04 µg/ml) on the absorbance of the colored product was studied in ethanol medium in the range of 1.0 - 7.0 ml at 70˚C. The absorbance increases with the increase in the volume of DHBA became constant at 6.0 ml. Further addition of DHBA did not cause change in absorbance and therefore 48.024 µg/ml DHBA was chosen as an optimum value (Figure 2(a)).
![]()
Scheme 1.Suggested reaction pathway between 2,5-Dihydroxy benzaldehyde (DHBA) and Gabapentin.
![]()
Figure 1. Absorption spectra of (1) 2.9 × 10−4 M GBP in ethanol, (2) 6.0 × 10−4 M DHBA reagent in ethanol and (3) GBP-DHBA reaction product, (CGBP = 2.9 × 10−4, CDHBA = 6.0 × 10−4 M). In ethanol at T = 75 ± 0.15˚C.
3.6. The Effect of Temperature
The effect of temperature on the absorbance of the reaction product was studied in ethanol medium in the range (45˚C - 80˚C), keeping the constant concentration of GBP (14.898 µg/ml) and (24.012 µg/ml) DHBA. The maximum absorbance was obtained at 75˚C ± 0.15˚C (Figure 2(b)).
3.7. The Influence of Time
The reaction time was determined by following the absorbance of the developed Schiff base in ethanol medium at 75˚C at different time intervals. Complete color development was attained after 15 min (Figure 2(c)).
3.8. Stoichiometry of the Reaction
The Stoichiometry of the GBP-DHBA Schiff base was further verified by the method of continuous variation [32] . In solution with CT = CG + CDHBA = 3.48 × 10−4 mol∙L−1 at 75˚C, the maximum of the Jop’s plot corresponds to a component ratio of 1:2 (GBP:DHBA).
3.9. Equilibrium Constant and Free Energy Change
The equilibrium Constant was determined for the interaction of gabapentin drug with DHBA reagent using Benesi-Hildebrand equation [33] .
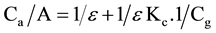
where Ca and Cg are the concentration of the aldehyde reagent and GBP drug respectively. A is the absorbance, ε is the molar absorptivity and Kc is the equilibrium Constant of GBP-DHBA Schiff base. The free energy change of reaction product (∆G) was calculated from the equilibrium constant by the following equation [34] .

where ∆G is the free energy change of the Schiff base (K∙cal∙mol−1), R is the gas constant (0.001987 K∙cal∙mol−1∙deg−1), T the temperature in Kelvin (273 + C0) and Kc is the equilibrium Constant of drug-DHBA reaction product.
The calculated values of equilibrium Constant Kc and free energy change of GBP-DHBA Schiff base were found to be 4.593 × 103 and −5.832 K∙cal∙mol−1 respectively.
3.10. Construction of the Calibration Curve and Statistical Analysis
Under the optimum experimental conditions, the standard calibration curve (Figure 3) for the proposed method
![]()
![]()
![]()
Figure 2. Reaction condition of the color formation of GBP-DHBA reaction product. (a) Effect of DHBA (by volume), GBP: 29.796 µg∙ml−1, T = 70˚C; (b) Effect of temperature, GBP: 14.898 µg∙ml−1, DHBA: 24.012 g∙ml−1; (c) Effect of time, GBP: 14.898 µg∙ml−1, DHBA: 24.012 µg∙ml−1, T = 75˚C.
![]()
Figure 3. Absorption spectra of GBP-DHBA reaction product, GBP concentration range from 2.483 (1) to 49.66 µg∙ml−1 (11) with regular successive additions in presence of 6.0 × 10−4 M DHBA reagent in ethanol at T = 75 ± 15˚C.
obeyed Beer’s law over the concentration range of 2.57 - 37.25 µg/ml at λmax = 445 nm. It was found that the absorbance was stable for at least two days at room temperature. Linear regression analysis of calibration data gave the regression equation cited in Table 1, with correlation coefficient close to unity.
The within day precision assay was carried out through replicate analysis (n = 3) of gabapentin corresponding to 15, 20, 30 µg/ml.
The interday precision was also evaluated through replicate analysis of the pure sample for three consecutive days at the same concentration levels as used in within day precision. The results of these assays are reported in Table 2.
As can be seen from Table 2 that recovery values for intraday and interday precision were in the range of 99.25% to 101.53% and RSD values for intraday and interday precision were in the range of 0.0057% to 0.018%.
3.11. Specificity
The specificity of the method was investigated by observing any interference encountered from the excipients generally presented in pharmaceutical formulations. The good percentage recoveries were summarized in Table 3 revealed that no interference was observed from any of these excipients in the proposed method.
3.12. Limit of Detection (LOD) and Limit of Quantification (LOQ)
The LOD and LOQ were calculated for GBP-DHBA reaction product. The theoretically determined values of LOD and LOQ were cross checked by actual analysis of these concentrations using proposed method (Table 1).
![]()
Table 1. Regression Analysis Data and summary of validation parameters for the proposed method.
![]()
Table 2. Summary of accuracy and precision results of the proposed method in pure form.
aMean for 3 independent analysis; bSAE, standard analytical error; cC.L., confidence limit at 95% confidence level and 4 Degrees of Freedom (t = 2.776).
3.13. Applications
The proposed spectrophotometric method was applied to the determination of gabapentin in pharmaceutical formulations (Gaptin and Conventin).
The results obtained from Table 3 suggested that the proposed method could be applied for the determination of gabapentin in its dosage forms without interference observed at concentration levels examined.
The excellent linearity of the calibration graphs was clearly evident by the values of the correlation coefficients and standard deviations as shown in Table 4.
3.14. Infrared Spectra
The I. R. spectra of gabapentin showed the expected doublet of primary NH2 group at 2857 and 2931 cm−1, C-N stretch at 1165 cm−1 and the carbonyl stretch of COOH group at 1615 cm−1. 2,5-dihydroxybenzaldehyde exhibited broad band at 3277.4 - 3250 cm−1 owing to two OH groups and C=O stretch of aldehyde group at 1652 cm−1.
The formation of the Schiff base was evidenced by comparing the I. R. spectra of GBP-DHBA reaction product with that of GBP and DHBA. It was found that the twin peaks of NH2 in GBP disappeared indicating that the primary amine has been changed to tertiary. Also the I.R. spectra of GBP-DHBA reaction product exhibits a strong band at 1622 cm−1 which is characteristic of the azomethin HC=N-group.
4. Conclusion
The proposed spectrophotometric method was found to be simple, selective, rapid and sensitive compared with other established spectrophotometric methods. The reagent utilized in the proposed method was cheep, readily available and the procedure did not involve any critical reaction conditions or tedious sample preparation. Moreover, the method was free from interference by common additives and excipients. Also this method required less time for analysis, provided better RSD and LOD and had a wide concentration range over the pre-
![]()
Table 3. Recovery of gabapentin in presence of different excipients.
![]()
Table 4. Application of the proposed method to the determination of gabapentin drug in dosage forms.
aProduct of Al-Debeiky Pharmaceutical products for Delta Pharma-Egypt; bproduct of EVA Pharma-Egypt.
viously published methods [35] [36] . Hence, it could be concluded that the developed spectrophotometric me- thod was accurate, precise, and selective and could be employed successfully for the estimation of gabapentin in pharmaceutical formulations. The excellent recoveries obtained showed no interference from the common excipients.
NOTES
*Corresponding author.