Heterocyclic Synthesis via Enaminones: Synthesis and Molecular Docking Studies of Some Novel Heterocyclic Compounds Containing Sulfonamide Moiety ()
1. Introduction
Enaminones are polydentate reagents that have been utilized extensively in this decade as building blocks in organic synthesis [1] -[6] . Also sulfonamides possess many types of biological activities and representatives of this class of pharmacological agents are widely used in clinic as antibacterial [7] , antithyroid, diuretic, hypoglycaemic and anti-cancer [8] -[12] . Moreover, non-steroidal anti-inflammatory drugs (NSAIDs) are widely employed in musculoskeletal disease, as well as their anti -inflammatory properties [13] . After widely evaluation, NSAIDs are efficacy in different clinical setting, and act as a COX inhibitor (COX-1 and COX-2) through inhibiting the production of prostaglandins (PGs) [14] -[16] . Diclofenac is a one from famous available members of this drug’s class under current clinical usage [17] , and suffers from a common toxicity of gastrointestinal drawback, due to inhibition non-selectivity of cyclooxygenase enzyme [18] -[20] , also, its display anti-microbial [21] -[23] , ulcerogenic, analgesic, anti-inflammatory, lipid peroxidation [24] [25] , antitumor [26] and inhibitor formation of transthyretin amyloid fibril properties [27] . In this paper, we have reported a variety of syntheses of heteroaromatics developed using functionally substituted enaminones as readily obtainable building blocks possessing multiple electrophilic and nucleophilic moieties.
2. Material and Methods
2.1. Experimental
All melting points, antioxidant and anticancer activities are uncorrected. IR spectra (KBr) were recorded on FT-IR 5300 spectrometer and Perkin Elmer spectrum RXIFT-IR system (ν, cm−1). The 1H-NMR spectra were recorded in (DMSO-d6) at 300 MHz on a Varian Mercury VX-300 NMR spectrometer (δ, ppm) using TMS as an internal standard. 13C-NMR spectra were recorded on Varian Mercury VX 300 NMR using DMSO-d6 as solvent and TMS as an internal standard. Mass spectrum was obtained on GC MS-QP 1000 EX mass spectrometer at 70 eV. Elemental analyses were carried out by the Microanalytical Research Center, Faculty of Science, Cairo University and Al-Azhar University.
(E)-4-(3-(Dimethylamino)acryloyl)-N, N-diethylbenze-nesulfonamide (2).
A mixture of 4-acetyl-N,N-diethylbenzenesulfonamide 1(0.01 mol) and DMF-DMA (0.012 mol) in dry xylene (50 ml) was heated under reflux for 4 hr. The separated solid was filtered off, washed with ethanol and recrystallized to give (2).Color: bright yellow; Yield: 83%; M.p.: 96˚C - 98˚C; FT-IR (KBr, ν, cm−1): 3100 (CH-arom.), 2920 (CH-aliph.), 1646 (CO), 1336, 1154 (SO2); 1HNMR (300 MHz, DMSO-d6, δ, ppm): 1.01 (t, 6H, CH3-CH2), 2.90 and 3.13 (2s, 6H, N(CH3)2), 3.29 (q, 4H, CH2-CH3), 5.79 and 7.72 (dd, 2H, olefinic CH = CH; J = 12.25 Hz), 7.78 and 8.02 (dd, 4H, AB-ArH; J = 8.4 Hz); 13C-NMR (300 MHz, DMSO-d6, δ, ppm):14.6, 40.3, 42.3, 91.4, 127.1, 128.5, 141.8, 144.1, 155.4, 184.7; Anal. Calcd. for C15H22N2O3S: C, 58.04; H, 7.14; N, 9.02. Found: C, 57.91; H, 7.03; N, 8.90.
4-(1-Acetyl-1H-pyrazol-3-yl)-N, N-diethylbenzenesulfonamide (4).
A mixture of enaminone 2(0.01 mol) and hydrazine hydrate (0.01 mol) in ethanol/acetic acid (30 ml) (1:1) was heated under reflux for 5hrs. During the reflux period, a crystalline solid was separated. The separated solid was filtered off, washed with ethanol to give 4. Color: White; Yield: 73% ; M.p.: 187˚C - 189˚C ; FT-IR (KBr, ν, cm−1): 1684 (CO), 1380, 1152 (SO2); 1HNMR (300 MHz, DMSO-d6, δ, ppm): 1.02 (t, 6H, CH3-CH2), 1.79 (s, 3H, CH3), 3.31 (q, 4H, CH2-CH3), 6.81 and 8.85 (dd, 2H,H- 4, H-5 pyrazole), 7.77 and 7.98 (dd, 4H, AB-ArH; J = 8.4 Hz); MS (EI, m/z (%)): 321 (M+)(8.6), 77 (100); Anal. calcd. for C15H19N3O3S: C, 56.06; H, 5.96; N, 13.07. Found: C, 55.92; H, 5.80; N, 12.93.
4-(1-(2-Cyanoacetyl)-1H-pyrazol-3-yl)-N,N-diethylbenzenesulfonamide (5).
A mixture of enaminone 2(0.01mol) and cyanoacetohydrazide (0.01 mol) in ethanol/acetic acid (30 ml) (1:1) was heated under reflux for 3hrs. During the reflux period, a crystalline solid was separated. The separated solid was filtered off, washed with ethanol to give 5. Color: yellow; Yield: 68%; M.p.: 215˚C - 217˚C; FT-IR (KBr, ν, cm−1): 2224 (CN), 1638 (CO), 1334, 1156 (SO2); 1HNMR (300 MHz, DMSO-d6, δ, ppm): 1.03 (t, 6H, CH3- CH2), 3.22 (q, 4H, CH2-CH3), 3.31 (s, 2H, CH2), 7.47 and 8.23 (dd, 2H,H-4, H-5 pyrazole), 7.96 and 8.30 (dd, 4H, AB-ArH; J = 8.4 Hz); Anal. calcd. for C16H18N4O3S: C, 55.48; H, 5.24; N, 16.17. Found: C, 55.33; H, 5.11; N, 16.01.
4-(4-Cyanobenzo[4,5]imidazo[1,2-a]pyridin-1-yl)-N,N-diethylbenzenesulfonamide (7).
A mixture of enaminone 2 (0.01 mol) and 1H-benzo-imidazole-2-ylacetonitrile (0.01 mol) in glacial acetic acid (30 ml) was refluxed for 2 hr. The solid product which obtained after cooling was collected by filtration and recrystallized to give 7. Color: yellowish white; Yield: 65%; M.p.: 332 - 334˚C; FT-IR (KBr, ν, cm−1): 2986 (CH-aliph.), 2228 (CN), 1334, 1156 (SO2); 1HNMR (300 MHz, DMSO-d6, δ, ppm): 1.08 (t, 6H, CH3-CH2), 3.38 (q, 4H, CH2-CH3), 7.02 - 8.33 (m, 10H, ArH + 2H pyridine ring); MS, m/z (%): 404 (M+)(20.4), 268 (100); 13C-NMR (300 MHz, DMSO-d6, δ, ppm): 14.3, 40.3, 103.2, 112.3, 114.7, 120.5, 122.0, 126.6, 128.0, 129.4, 130.4, 137.1, 138.1, 144.8, 160.9; Anal. calcd. for C22H20N4O2S: C, 65.33; H, 4.98; N, 13.85. Found: C, 65.20; H, 4.86; N, 13.71.
4-(8-Oxo-6-thioxo-7,8-dihydro-6H-pyrimido[1,6-a]pyrimidin-4-yl)-N,N-diethylbenzenesulfonamide (8).
A mixture of enaminone 2 (0.01mol) and 6-amino-2-thiouracil (0.01 mol) in glacial acetic acid (40 ml) was refluxed for 3 h. The solvent was removed by distillation under reduced pressure and the resulting solution was left to cool. The solid precipitate was collected by filtration; Color: yellow; Yield: 69%; M.p.: 315˚C - 317˚C; FT-IR (KBr, ν, cm−1): 3110 (NH), 2978 (CH-aliph.), 1686 (CO), 1336, 1158 (SO2); 1HNMR (300 MHz, DMSO-d6, δ, ppm): 1.02 (t, 6H, CH3-CH2), 1.90 (s, 1H, SH), 3.18 (q, 4H, CH2-CH3), 7.93 - 8.97 (m, 7H, ArH + pyrimidine ring); MS (EI, m/z (%)):390 (M+)(29.2), 254 (83.3), 56 (100); Anal. calcd. for C17H18N4O3S2: C, 52.29; H, 4.65; N, 14.35. Found: C, 52.14; H, 4.51; N, 14.22.
4-([1,2,4]Triazolo[4,3-a]pyrimidin-5-yl)-N,N-diethylbenzenesulfonamide (10).
A mixture of enaminone 2 (0.01 mol) and 3-amino-1H-1, 2,4-triazole (0.01 mol) in acetic acid (30 ml) was refluxed for 5 hr. During the reflux period, a crystalline solid was separated. The separated solid was filtered off, washed with ethanol and recrystallized to give the compound 10. Color: yellow; Yield: 73%; M.p.: 238˚C - 240˚C; FT-IR (KBr, ν, cm−1): 2980 (CH-aliph.), 1336, 1160 (SO2); 1HNMR (300 MHz, DMSO-d6, δ, ppm): 1.05 (t, 6H, CH3-CH2), 3.20 (q, 4H, CH2-CH3), 7.67 and 8.97 (dd, 2H, pyrimidine ring; J6,7 = 4.6 Hz ), 7.98 and 8.28 (dd, 4H, AB-ArH; J = 8.4 Hz ), 8.72 (s, 1H, CH Triazole); Anal. calcd. for C15H17N5O2S: C, 54.36; H, 5.17; N, 21.13. Found: C, 54.22; H, 5.04; N, 21.04.
General procedure for preparation of (13a,b).
A mixture of enaminone 2 (0.01 mol) and 5-amino-3-(methylthio)-1H-pyrazole-4-carbonitrile or 3-phenyl- 1H-pyrazol-5-amine (0.01 mol) in ethanol (60 ml) was heated under reflux for 5 hr. During the reflux period, a crystalline solid was separated. The separated solid was filtered off, washed with ethanol and recrystallized from the appropriate solvents to give (13a,b).
4-(3-Cyano-2-(methylthio)pyrazolo[1,5-a]pyrimidin-7-yl)-N,N-diethylbenzenesulfonamide (13a).
Color: brownish yellow; Yield: 75%; M.p.: 265 - 267˚C; FT-IR (KBr, ν, cm−1): 2976 (CH-aliph.), 2220 (CN), 1352, 1154 (SO2); 1HNMR (300 MHz, DMSO-d6, δ, ppm): 1.04 (t, 6H, CH3-CH2), 2.68 (s, 3H, SCH3), 3.21 (q, 4H, CH2-CH3), 7.54 and 8.82 (dd, 2H, pyrimidine ring; J5,6 = 4.6 Hz ), 7.98 and 8.28 (dd, 4H, AB-ArH; J = 9.1 Hz ); MS (EI, m/z (%)):401 (M+)(26.4), 265 (100); Anal. calcd. for C18H19N5O2S2: C, 53.85; H, 4.77; N, 17.44. Found: C, 53.71; H, 4.62; N, 17.31.
4-(2-Phenylpyrazolo[1,5-a]pyrimidin-7-yl)-N,N-diethylbenzenesulfonamide (13b).
Color: yellow; Yield: 68%; M.p.: 150˚C - 152˚C; FT-IR (KBr, ν, cm−1): 2970 (CH-aliph.), 1352, 1154 (SO2); MS (EI, m/z (%)):406 (M+)(32.6), 270 (100); Anal. calcd. for C22H22N4O2S: C, 65.00; H, 5.46; N, 13.78. Found: C, 64.85; H, 5.32; N, 13.64.
4-(5-Hydroxybenzofuran-3-carbonyl)-N,N-diethyl-benzenesulfonamide (17).
To a stirred solution of enaminone (1; 0.01 mol) in glacial acetic acid (30 ml), 1,4-benzoquionone (0.01 mol) was added, stirring was continued for 7 hr. At room temperature, the reaction mixture was evaporated in vacuo and the solid product was isolated by filtration and recrystallized to give (17). Color: white; Yield: 78%; M.p. 235˚C - 237˚C; FT-IR (KBr, ν, cm−1): 3320 (OH), 1614 (CO), 1340, 1156 (SO2); 1HNMR (300 MHz, DMSO-d6, δ, ppm): 1.03 (t, 6H, CH3-CH2), 3.19 (q, 4H, CH2-CH3), 6.84 and 7.52 (dd, 2H, H-6,7 benzofuran), 7.49 (s, 1H, H-4 benzofuran), 7.93-8.02 (dd, 4H, AB-ArH; J = 7.65 Hz), 8.62 (s, 1H, H-2 benzofuran), 9.52 (s, 1H, OH exchangeable with D2O); 13C-NMR (DMSO-d6) δ: 14.7, 40.3, 107.0, 112.6, 115.2, 125.8 127.6, 130.0, 142.3, 143.3, 149.5, 155.4, 156.1, 191.4. Anal. Calcd. for C19H19NO5S: C, 61.11; H, 5.13; N, 3.75. Found: C, 61.05; H, 5.09; N, 3.70.
N-(6-(4-(N,N-diethylsulfamoyl)phenyl)-2-oxo-2H-pyran-3-yl)benzamide (19).
A mixture of enaminone 2 (0.01 mol) and benzoylglycine (0.01 mol) in acetic anhydride (30 ml) was heated under reflux for 2 hr. The reaction mixture was concentrated in vacuo. The solid product which formed upon cooling was filtered off then washed with ethanol. Color: white; Yield:73%; M.p.: 255˚C - 257˚C; FT-IR (KBr, ν, cm−1): 3336 (NH), 2972 (CH-aliph.), 1700 and 1660 (2CO), 1334, 1154 (SO2); 1HNMR (300 MHz, DMSO-d6, δ, ppm): 1.02 (t, 6H, CH3-CH2), 3.15 (q, 4H, CH2-CH3), 7.32 and 8.22 (dd, 2H, pyranone; J = 7.6 Hz), 7.53 - 8.03 (m, 9H, ArH), 9.69 (s, 1H, NH); 13C-NMR (300 MHz, DMSO-d6, δ, ppm): 14.6, 40.2, 91.1, 109.1, 126.1, 128.2, 129.1, 129.3, 135.2, 140.1, 158.2, 163.2, 163.6; Anal. calcd. for C22H22N2O4S: C, 64.37; H, 5.40; N, 6.82. Found: C, 64.24; H, 5.25; N, 6.68.
General Procedure for the Reaction of 2 with Active Methylene Compounds to Form ( 20a,b) and (21).
Sodium ethoxide solution (0.23 g sodium metal in 25 ml absolute ethanol) was added with stirring to a mixture of 2 (0.01 mol) and cyanoacetamide, cyanothioacetamide and malononitrile dimer (0.01 mol) in absolute ethanol (25 ml). The reaction mixture was refluxed for 3 h, then poured into cooled water (50 ml) and neutrallized with diluted hydrochloric acid. The precipitate that formed was filtered off, dried, and crystallized from the appropriate solvents to give:
4-(5-Cyano-6-oxo-1,6-dihydropyridin-2-yl)-N,N-diethylbenzenesulfonamide (20a).
Color: yellow; Yield: 77%; M.p.: 220˚C - 222˚C; FT-IR (KBr, ν, cm−1): 2926(CH-aliph.), 2226 (CN), 1648 (CO), 1332, 1156 (SO2); 1HNMR (300 MHz, DMSO-d6, δ, ppm): 1.02 (t, 6H, CH3-CH2), 3.19 (q, 4H, CH2-CH3), 7.87 - 8.47 (m, 7H, ArH + NH); MS (EI, m/z (%)): 331 (M+)(13.8), 64 (100); Anal. calcd. for C16H17N3O3S: C, 57.99; H, 5.17; N, 12.68. Found: C, 57.84; H, 5.03; N, 12.55.
4-(5-Cyano-6-mercaptopyridin-2-yl)-N,N-diethylbenzenesulfonamide (20b).
Color: yellowish white; Yield: 65%; M.p.: 234˚C - 236˚C; FT-IR (KBr, ν, cm−1): 3114 (NH), 2938 (CHaliph.), 2224 (CN), 1334, 1154 (SO2); MS (EI, m/z (%)): 347 (M+)(24.1), 84 (100); Anal. calcd. for C16H17N3O2S2: C, 55.31; H, 4.93; N, 12.09. Found: C, 55.17; H, 4.80; N, 11.95.
4-(5-Cyano-6-(dicyanomethylene)-1,6-dihydropyridin-2-yl)-N,N-diethylbenzenesulfonamide (21).
Color: yellow; Yield: 68%; M.p.: 296˚C - 298˚C; FT-IR (KBr, ν, cm−1): 3240 (NH), 2214 (CN), 1328, 1156 (SO2); MS (EI, m/z (%)): 379 (M+) (12.2), 55 (100); Anal. calcd. for C19H17N5O2S: C, 60.14; H, 4.52; N, 18.46. Found: C, 60.01; H, 4.37; N, 18.32.
General procedure for preparation of (23a,b).
To a mixture of enaminone 2 (0.01 mol) and thiourea or guanidine hydrochloride (0.01 mol) in ethanol (40 ml) was added a few drops of piperidine as catalyst. The reaction mixture was refluxed for 4 hr, then poured into cold water (50 ml) and neutralized with diluted hydrochloric acid. The precipitate that formed was filtered off, dried, and crystallized from the appropriate solvents to give:
4-(2-Thioxo-1,2-dihydropyrimidin-4-yl)-N,N-diethyl-benzenesulfonamide (23a).
Color: faint brown; Yield: 82%; M.p.: 190˚C - 192˚C; FT-IR (KBr, ν, cm−1): 3126 (NH), 2976 (CH-aliph.), 1336, 1164 (SO2); 1HNMR (300 MHz, DMSO-d6, δ, ppm): 1.05 (t, 6H, CH3-CH2), 3.17 (q, 4H, CH2-CH3), 7.47 and 8.16 (dd, 2H, pyrimidine ring; J = 6.6 Hz ), 7.93 and 8.31 (dd, 4H, AB-ArH; J = 8.4 Hz ), 13.8 (s, 1H, NH); MS (EI, m/z (%)):323 (M+)(25.5), 308 (78.4), 187 (100); Anal. calcd. for C15H17N5O2S: C, 54.36; H, 5.17; N, 21.13. Found: C, 54.22; H, 5.04; N, 21.02.
4-(2-Aminopyrimidin-4-yl)-N,N-diethylbenzenesulfonamide (23b).
Color: yellow; Yield: 75%; M.p.: 175˚C - 177˚C; FT-IR (KBr, ν, cm−1): 3478, 3300 (NH2), 3162 (CH-arom.), 2980 (CH-aliph.), 1332, 1152 (SO2); MS (EI, m/z (%)): 306 (M+)(19.0), 291 (62.0), 170 (100); Anal. calcd. for C14H18N4O2S: C, 54.88; H, 5.92; N, 18.29. Found: C, 54.73; H, 5.80; N, 18.15.
General procedure for preparation of (24a,b).
A mixture of enaminone 2 (0.01 mol) and p-toluidine or p-phenitidine (0.01 mol) in a mixture of ethanol/acetic acid (50 ml) (1:1) was heated under reflux for 3 hr. During the reflux period, a crystalline solid was separated. The separated solid was filtered off, washed with ethanol and recrystallized from the appropriate solvents to give:
4-(3-(p-Tolylamino)acryloyl)-N,N-diethylbenzenesulfonamide (24a).
Color: yellow; Yield: 88%; M.p.: 265˚C - 267˚C; FT-IR (KBr, ν, cm−1): 3176 (NH), 2982 (CH-aliph.), 1644 (CO), 1334, 1154 (SO2); 1HNMR (300 MHz, DMSO-d6, δ, ppm): 1.02 (t, 6H, CH3-CH2), 2.25 (s, 3H, CH3), 3.16 (q, 4H, CH2-CH3), 6.09 (d, 1H, COCH, J = 7.6 Hz), 7.94 (m, 1H, CH-NH), 7.07-7.15 (dd, 4H, AB-ArH; J = 8.4 Hz), 7.85 - 7.96 (dd, 4H, AB-ArH; J = 8.4 Hz), 12.10 (d, 1H, NH exchangeable with D2O); Anal. calcd. for C20H24N2O3S: C, 64.49; H, 6.49; N, 7.52. Found: C, 64.32; H, 6.35; N, 7.40.
4-(3-((4-Ethoxyphenyl)amino)acryloyl)-N,N-diethylbenzenesulfonamide (24b).
Color: reddish yellow; Yield: 85%; M.p.: 280˚C - 282˚C; FT-IR (KBr, ν, cm−1): 3170 (NH), 2980 (CH-aliph.), 1628 (CO), 1342, 1156 (SO2); 1HNMR (300 MHz, DMSO-d6, δ, ppm): 1.01 (t, 6H, CH3-CH2), 1.27 (t, 3H, CH3-CH2O), 3.15 (q, 4H, CH2-CH3), 3.97 (q, 2H, OCH2-CH3), 6.06 (d, 1H, COCH, J = 7.8 Hz), 7.92 (m, 1H, CH-NH ), 6.89 - 7.26 (dd, 4H, AB-ArH; J = 8.4 Hz), 7.83 - 8.06 (dd, 4H, AB-ArH; J = 8.4 Hz), 12.17 (d, 1H, NH exchangeable with D2O); Anal. calcd. for C21H26N2O4S: C, 62.66; H, 6.51; N, 6.96. Found: C, 62.51; H, 6.36; N, 6.82.
4-(3-Benzoyl-1-phenyl-1H-pyrazole-4-carbonyl)-N,N-diethylbenzenesulfonamide (30).
To a mixture of enaminone 2 (0.01 mol) and the hydrazonoyl bromide (85; 0.01 mol) in benzene (40 ml) an equivalent amount of triethylamine was added. The reaction mixture was heated under reflux for 2 hr. the solvent was distilled at reduced pressure and the residual viscous liquid was taken in ethanol then the resulting solid was collected by filtration, washed thoroughly with ethanol, dried and finally recrystallized to give the compound 30. Color: yellow; Yield: 81%; M.p.: 224˚C - 226˚C; FT-IR (KBr, ν, cm−1): 2976 (CH-aliph.), 1660 (CO), 1336, 1154 (SO2); 1HNMR (300 MHz, DMSO-d6, δ, ppm): 1.03 (t, 6H, CH3-CH2), 3.14 (q, 4H, CH2-CH3), 7.21 - 8.41 (m, 15H, ArH + CH pyrazole); MS (EI, m/z (%)): 478 (M+)(8.5), 351 (18.9.3), 76 (100); Anal. calcd. for C27H25N3O4S: C, 66.51; H, 5.17; N, 8.62. Found: C, 66.37; H, 5.04; N, 8.48.
4-(2,7-Diphenyl-2H-pyrazolo[3,4-d]pyridazin-4-yl)-N,N-diethylbenzenesulfonamide (31).
A mixture of pyrazole derivative 30 (0.01 mol) and hydrazine hydrate (0.012 mol) in ethanol (50 ml) was heated under reflux for 4 hr. The separated solid was filtered off, washed with ethanol and recrystallized to give 31. Color: brownish yellow; Yield: 65%; M.p.: 292˚C - 294˚C; FT-IR (KBr, ν, cm−1): 2974 (CH-aliph.), 1332, 1160 (SO2); MS (EI, m/z (%)):483 (M+) (37.6), 347 (100); Anal. calcd. for C27H25N5O2S: C, 67.06; H, 5.21; N, 14.48. Found: C, 66.92; H, 5.08; N, 14.33.
2.2. Molecular Modeling Study
2.2.1. Generation of Ligand and Enzyme
Structures. Selection of COX structures.
Docking study was carried out for the target compounds into COX-1 (ID: 3N8Y) and COX-2 (ID: 1PXX) using MVD, 4.0 and MOE, 10. The crystal structure of the (COX) complexes with (1), which a selective inhibitor of COX-2 in co-crystallized form in the active site of the receptor. From X-ray crystal structure studies of the COX enzyme, the mouse enzyme is expected to be very similar to the human [28] , and can be used as a model for human COX enzyme.
2.2.2. Preparation of Small Molecule
Molecular modeling of the target compounds were built using MOE, and minimized their energy with PM3 through MOPAC. Our compounds were introduced into the (COX) binding site accordance the published crystal structures of (1) bound to the kinase.
2.2.3. Stepwise Docking Method
MOE Stepwise The crystal structure of the (COX) with a Diclofenac (1) as inhibitor molecule, was used in the receptor molecule, water and inhibitor molecules were removed, and hydrogen atoms were added. The parameters and charges were assigned to the MMFF94x force field. After alpha-site spheres were generated using the SITE FINDER module of MOE. The optimized 3D structures of molecules were subjected to generate different poses of ligands using triangular matcher placement method, which generating poses by aligning ligand triplets of atoms on triplets of alpha spheres representing in the receptor site points, a random triplet of alpha sphere centers is used to determine the pose during each iteration. The pose generated was rescored using London dG scoring function. The poses generated were refined with MMFF94x forcefield, also, the solvation effects were treated. The Born solvation model (GB/VI) was used to calculate the final energy, and the finally assigned poses were assigned a score based on the free energy in kJ/mol
3. Results and Discussion.
3.1. Chemistry
Treatment of 4-acetyl-N,N-diethylbenzenesulfonamide (1) with dimethylformamid-dimethylacetal (DMF-DMA) in dry dioxane afforded (E)-4-(3-(dimethylamino) acryloyl)-N,N-diethylbenzenesulfonamid (2) in high yield. (Scheme 1). The structure of the enaminone 2 was confirmed on the basis of elemental analysis and spectral data. Thus, the IR spectrum of compound 2 revealed absorption bands at (v cm−1): 2910 (CH-aliph.) and 1646 (C = O), while its 1H-NMR (δ ppm) spectrum (DMSO) indicated signals at: 2.90 and 3.13 (2s, 6H, N(CH3)2), 5.79 and 7.72 (dd, 2H, olefinic CH = CH; J = 12.25 Hz), which support that the structure in (E-form), not (Z-form). The reactivity of compound 2 towards some nitrogen nucleophiles was investigated. Thus, enaminone 2 was treated with hydrazine hydrate in refluxing ethanol/acetic, to produce intermediates 3 followed by acetylation to give 4-(1-acetyl-1H-pyrazol-3-yl)-N,N-diethylbenzenesulfonamide (4). In the same manner, the enaminone 2 reacted with 2-cyanoacetic acid hydrazide under the same experimental reaction conditions to afford 4-(1-(2- cyanoacetyl)-1H-pyrazol-3-yl)-N,N-diethylbenzenesulfonamide (5) (Scheme 1).
When compound 2 was allowed to react with active methylene reagent like: 2-cyanomethylbenzoimidazole in

Scheme 1. SSynthesis of pyrazole derivatives.
glacial acetic acid afforded 4-(4-cyanobenzo[4,5]imidazo [1,2-a] pyridin-1-yl)-N,N-diethylbenzenesulfonamide (7). Formation of 7 is assumed to proceed via the initial nucleophilic addition of the active methylene to enaminone double bond to afford the non-isolable intermediate 6 followed by elimination of water and dimethylamine (Scheme 2). On the other hand, the enaminone 2 condensed with 6-amino-2-thiouracil in glacial acetic acid at reflux temperature to afford N,N-diethyl-4-(8-oxo-6-thioxo-7,8-dihydro-6H-pyrimido[1,6-a] pyrimidin-4-yl) benzenesulfonamide (8), (Scheme 2). Enaminone 2 reacted with 3-amino-1H-1,2,4-triazole in acetic acid under reflux to afford [1,2,4]triazolo[4,3-a]pyrimidine derivatives (10) via the addition of the exoamino group of aminotriazole to α,β-unsaturated moiety in compound 2 followed by elimination of dimethylamine molecule to yield the corresponding acyclic non-isolable intermediate , which undergoes intramolecular cyclization by elimination of water molecule to afford the final product 10 not 9 (Scheme 2).
In the same manner, the enaminone 2 reacted with another heterocyclic amine like: 5-amino-3-methyl thiopyrazole-4-carbonitrile [29] [30] and 5-Phenyl-2H-pyrazol -3-yl amine under the same experimental reaction conditions to afford pyrazolo [1,5-a]pyrimidine derivatives 13a,b. The formation of 13a,b was assumed to takes place via the addition of exoamino group of aminopyrazole to α,β-unsaturated moiety of enaminone 2 to yield the corresponding acyclic non-isolable intermediate 12a,b which undergoes intramolecular cyclization by the elimination of the dimethylamine and water molecules to afford the final product 13a,b not 11a,b (Scheme 3).
Furthermore, Compound 2 reacted with 1,4-benzoquin-one in glacial acetic acid at room temperature to yield a product which formulated as N,N-diethyl-4-(5-hydroxy-benzofuran-3-carbonyl)benzenesulfonamide (17). Elucidation of structure 17 and refusing of structure 15 was based on 1H-NMR spectrum which indicates the disappearance of aldehydic signal and showed singlet signal at 9.52 ppm for H-2 benzofuran moiety. It's believed that electron rich (C-2) in the enaminone 2 initially adds to the activated double bond in the quinone yielding acyclic intermediate 16 which then cyclizes into 17 via dimethylamine elimination, and not afforded 15 (Scheme 4).
Enaminone 2 reacted with hippuric acid in acetic anhydride to give a product that was identified as N-(6-(4- (N,N-diethylsulfamoyl)phenyl)-2-oxo-2H-pyran-3-yl)benzamide (19), which confirmed on the basis of elemental analysis and spectral data, (Scheme 5). The formation of compound 19 was assumed to proceed via initial cyclization of hippuric acid into oxazolone derivative which then added to the activated double bond system of enaminone yielding 18 followed by intramolecular cyclization and rearrangement to give the final structure 19 (Scheme 5).
The reaction of 2 with some active nitriles such as cyanoacetamide, cyanothioacetamide and malononitrile dimer in sodium ethoxide was studied. Enaminones 2 were reacted with cyanoacetamide, cyanothioacetamide in refluxing ethanolic sodium ethoxide to give 4-(5-cyano-6-substituted pyridin-2-yl)-N,N-diethyl benzenesulfonamide 20a,b, (Scheme 6). Structures 20a,b were based on the correct elemental analyses and spectral data. Compound 2 was also reacting with malononitrile dimmer in refluxing ethanolic sodium ethoxide to give 4-(5-cyano-6-(dicyanomethylene)-1,6-dihydropyridin-2-yl)-N,N-diethylbenzenesulfonamide (21). All analytical and spectral data supported the suggested structure. The IR spectra showed an absorption band at 2214 (CN) cm−1.
Enaminone 2 reacted with guanidine hydrochloride and thiourea in refluxing ethanol in the presence of pipridine to give 23a,b. A plausible mechanism for the formation of compounds 23a,b is outlined in (Scheme 7). Which then undergo intramolecular cyclization and subsequent aromatization via the elimination of dimethylamine and water molecules under the reaction conditions to give 23a,b as depicted in (Scheme 7). On the other

Scheme 2. Synthesis of benzoimidazo pyridine, pyrimido and triazolo pyrimidine derivatives.

Scheme 3. Synthesis of pyrazolo pyrimidine derivatives.
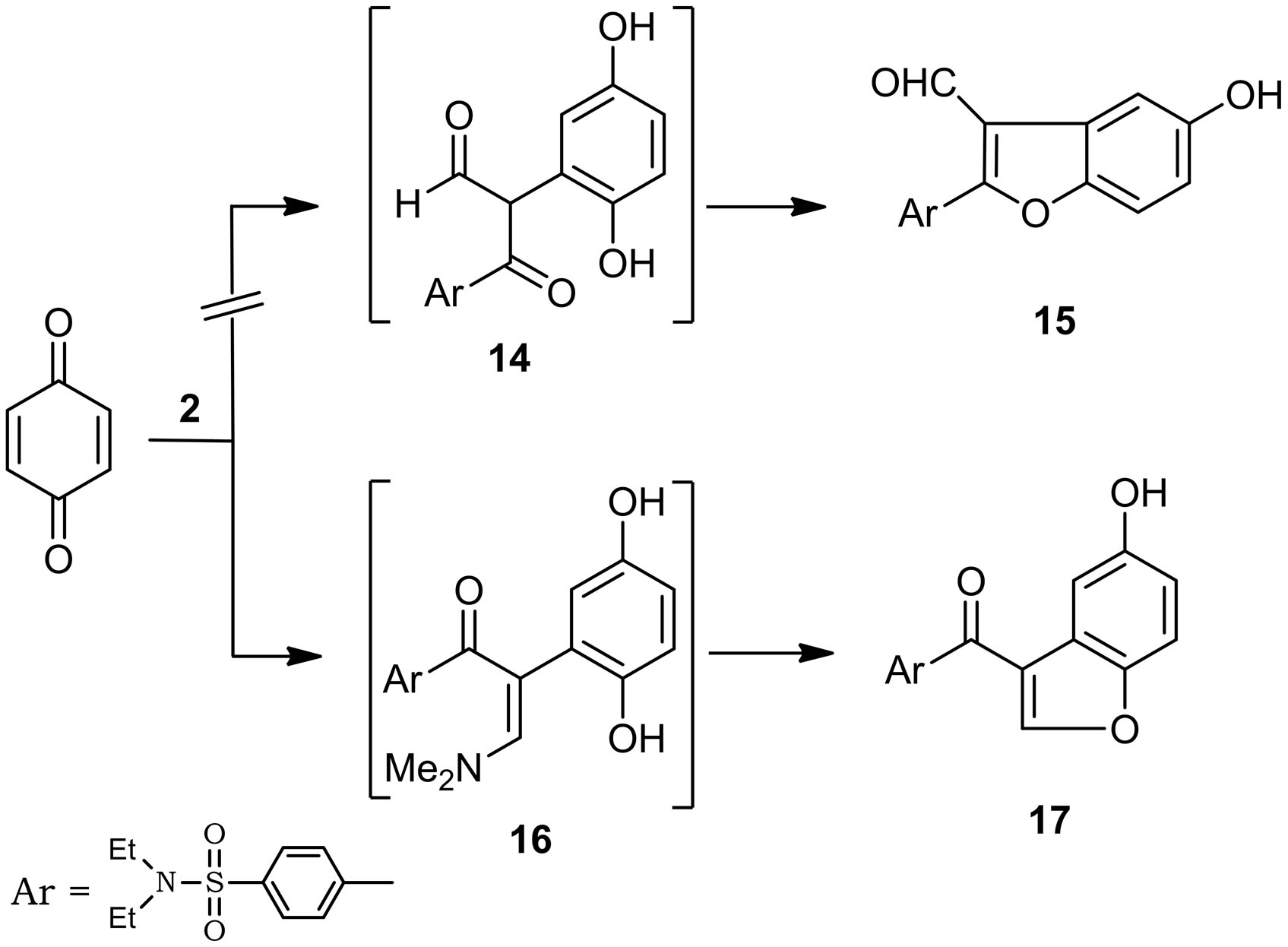
Scheme 4. Synthesis of benzofuran derivative.

Scheme 5. Synthesis of pyranone derivative.

Scheme 6. Synthesis of pyridine derivative.
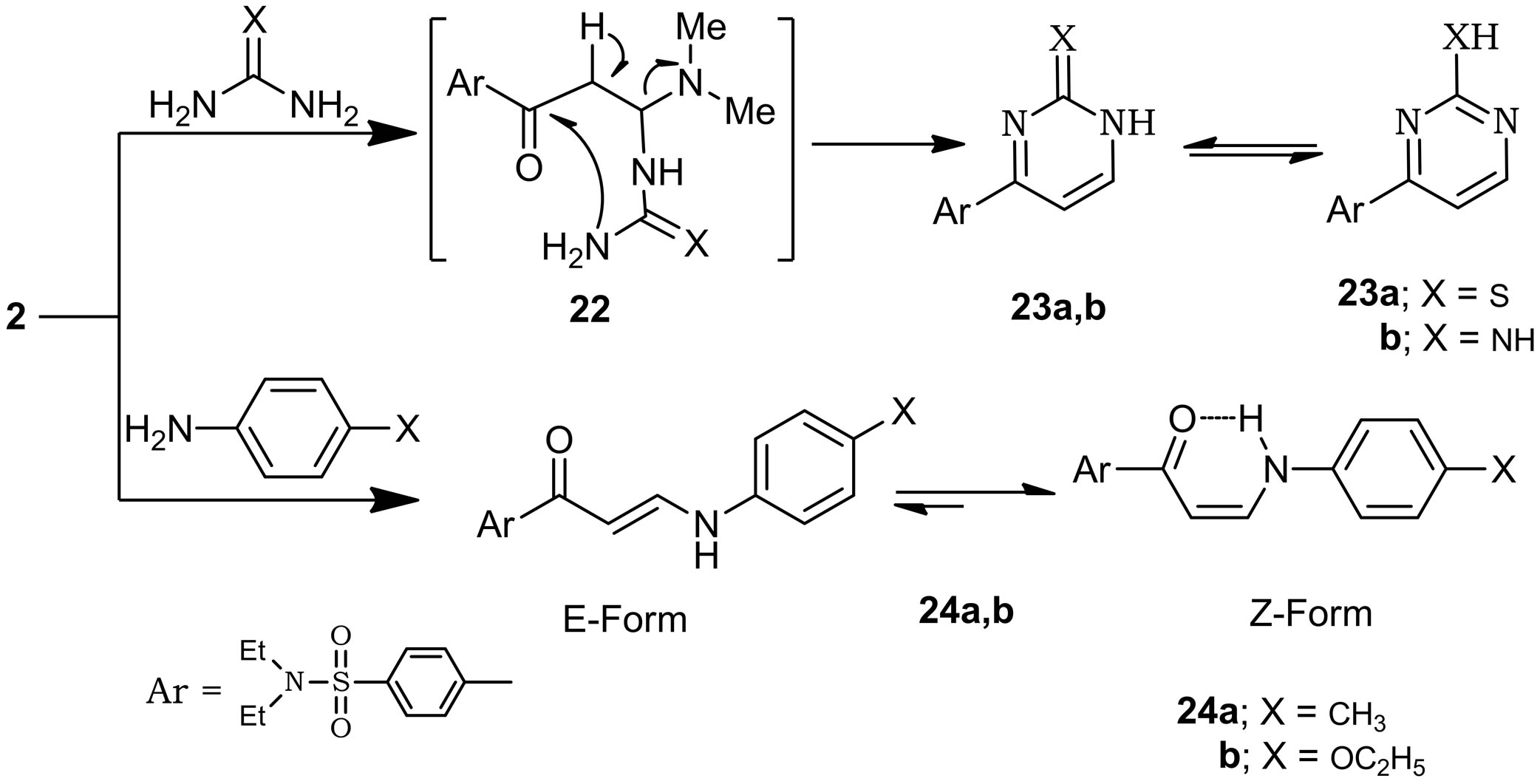
Scheme 7. Synthesis of pyrimidine derivatives.
hand, when enaminone (2) was treated with primary aromatic amines namely (p-toluidine and p-phenitidine) in a mixture of ethanol/acetic acid at reflux temperature afforded 4-(3-((p-substituted amino)acryloyl)-N,N-diethylbenzene sulfonamides (24a,b) (Scheme 7). The 1H-NMR spectrum of compounds 24a,b support this structures is (Z-form) not (E-form), where the coupling constant of the doublet signals for olefinic protons equal to 7.6, 7.8 Hz, respectively. Stabilization of (Z-form) is achieved by intramolecular hydrogen bonding (Scheme 7).
Hydrazonyl halides [31] -[33] has been reported to add to α,β-unsaturated carbonyl compounds to yield a mixture of isomeric pyrazolines [34] [35] . In the present work the reaction of enaminone 2 with nitrileimine 26 (generated in situ from the treatment of the hydrazonoyl bromide 25 with triethylamine in refluxing m-xylene) gave only one isolable product (TLC). From which two proposed structures 28 or 30 seemed possible (Scheme 8). The other possible regioisomer 28 was excluded on the basis of the spectral data of the isolated products. For example, in the pyrazole ring system, C-4 is the most electron-rich carbon, thus H-4 is expected to appear in the 1H-NMR spectra at higher field, typically near 6.0 ppm. On the other hand, H-5 is linked to the carbon attached

Scheme 8. Synthesis of pyrazolo pyridazine derivative.
to a nitrogen atom and thus it is deshielded to appear near 8.0 ppm. 1H-NMR spectrum of 30 exhibits a singlet at 8.41 ppm, which indicates the presence of the pyrazole H-5 rather than H-4 [36] . Pyrazole derivative 30 was assumed to be formed via initial 1, 3-dipolar cycloaddition of nitrileimine 26 to the activated double bond in compound 2 forming non isolable intermediate 29 followed by the loss of dimethylamine (Scheme 8). Interaction of pyrazole derivative 30 with hydrazine hydrate in refluxing ethanol absolute afforded pyrazolo[3,4-d] pyridazine derivative (31), which confirmed by elemental analysis and spectral data (Scheme 8).
3.2. Docking Studies
In brief, two isoforms of COX protein are known: COX-1 is responsible for the physiological production of prostaglandins, which is expressed in most tissues; and COX-2, is responsible for the increasing production of prostaglandins during process of inflammation, which is induced by endotoxins, cytokines and mitogens in inflammatory cells [37] . Recently, from analysis of X-ray cocrystal of arachidonic acid with COX-2 showed that, carboxylate coordinated with Tyr-385 and Ser-530 [38] , as well as the action of NSAIDs, through the interaction carboxylate group with Tyr-385 and Ser-530, which stabilize the negative charge of the tetrahedral intermediate [39] , and demonstrated that, Tyr-385 and Ser-530 have a structural and functional evidence for the importance of them in the chelating of the ligands [40] . Molecular docking of the synthesized compounds into the active site of COX was performed, in order to obtain biological data on a structural basis, through rationalized ligand-protein interaction behavior. All calculations for docking experiment were performed with MOE 2008.10 [41] . The tested compounds were evaluated in silico (docking), using X-ray crystal structures of COX-2 (ID: 4COX). The tested compounds were docked into active sites of both enzymes COX-2. The active site of the enzyme was defined to include residues within a 10.0 Å radius to any of the inhibitor atoms. The scoring function of the most stable docking model for testing compounds was applied to evaluate the binding affinities of the inhibitors complexes with (COX) active site (Table 1). The complexes were energy-minimized with an MMFF94 force field [42] till the gradient convergence 0.05 kcal/mol was reached. The active compounds docked successfully into the COX-2 active site. The compounds 2.10 and 17 in COX-2 active site exhibited, binding scores (−98.09, −118.71 and −125.06) Kcal/mol, respectively.
Structure activity relationships:
In order to get a deeper insight into the nature and type of interactions of docked compounds, the complexes between each compound and COX-2 receptor were visualized, and depicted in (Figures 1-3). Since, the H bonds play an important role in the structure and function of biological molecules, the current ligand-receptor interactions were analyzed on the basis of H bonding. In order to reduce the complexity, hydrophobic and π-cation interactions (>6 Å) are not shown in the Figures 1-3.
On COX-2 binding site (Figure 3); i-Compound 2 arranged in the binding pocket by adjusting phenyl ring

Table 1. Pharmacokinetic parameters important for good oral bioavailability of most active compounds.
TPSA: Polar surface area (A2); %ABS: Absorption percentage; Vol: Volume (A3); HBA: Number of hydrogen bond acceptor; HBD: Number of hydrogen bond donor; V: Number of violation from Lipinski’s rule of five; Log P: Calculated lipophilicity; Log S: Solubility parameter; mr: Molar Refractivity; ∆E: Energy Gaps (ev); η: Hardness (ev); S: Softness (ev); χ : Electronegativity (ev); σ: chemical potential (ev); ω: Electrophilicity (ev).
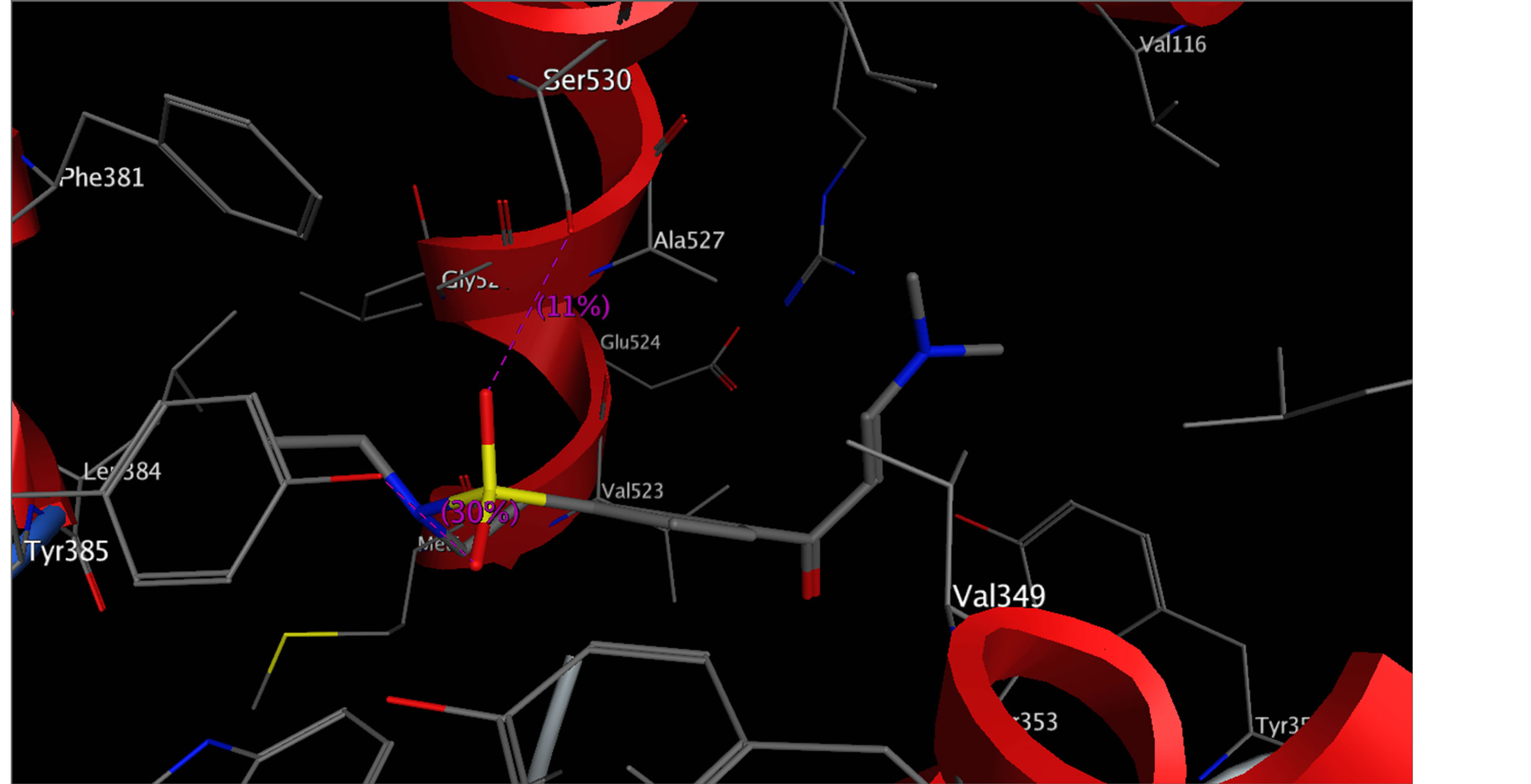
Figure 1. Docking compound 2 into the active site COX-2 with highest docking score H-bonds are in pink.
perpendicular with N-ethyl and formed an electrostatic bond with Ser-530 and Tyr-385; ii-Compound 10 and 17, were interacted with different modes with binding pocket, by forming two strong hydrogen bond interaction with important residue Arg-120, which explain increasing of the binding energy of its compounds. The results obtained clearly reveals that, the amino acid residues close to the reference molecules are mostly the same as those observed in the most active compounds complexes with protein (Figures 2 and 3). These results indicate that, the compounds 2, 10 and 17 act as selective inhibitors against COX-2.
ADMET factors profiling:
Oral bioavailability was considered to play an important role in the development of bioactive molecules as

Figure 2. Docking compound 10 with a highest docking score into the active site COX-2.
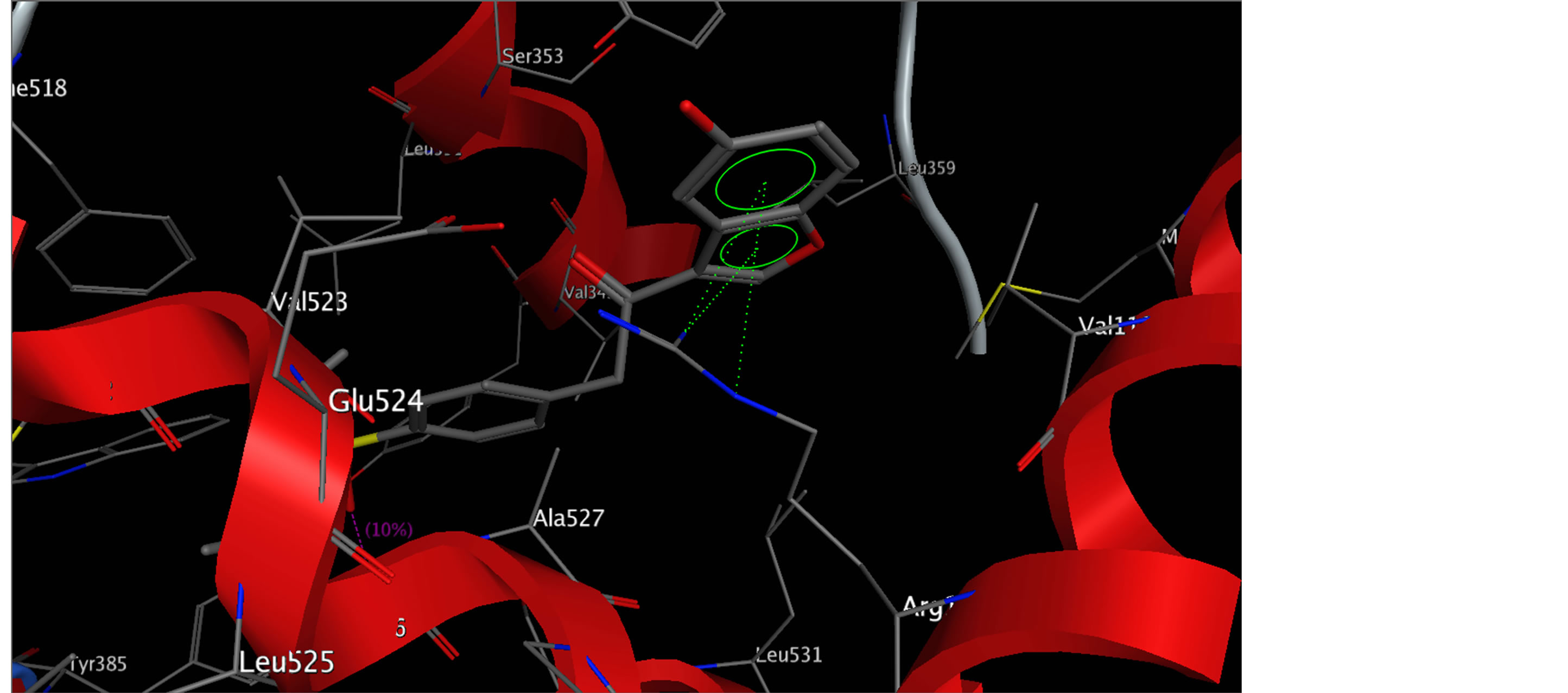
Figure 3. Docking compound 17 with a highest docking score into the active site COX-2.
therapeutic agents. Many potential therapeutic agents fail to reach the clinic, because of their ADMET (absorption, distribution, metabolism, elimination and toxic) Factors. Therefore, a computational study for prediction of ADMET properties of the molecules was performed for most active compounds, by the determination of topological polar surface area (TPSA), a calculated percent absorption (%ABS) which was estimated by Zhao et al. equation [29] , and “rule of five” formulated by Lipinski [30] , which established that, chemical compound could be an orally active drug in humans, if no more than one violation of the following rule:
1) ClogP (partition coefficient between water and octanol) < 5.
2) Number of hydrogen bond donor sites ≤ 5.
3) Number of hydrogen bond acceptor sites ≤ 10, iv), molecular weight < 500.
In addition, the total polar surface area (TPSA) is another key property linked to drug bioavailability, the passively absorbed molecules with (TPSA > 140) have low oral bioavailability [43] . All calculated descriptors were performed using MOE Package, and their results were disclosed in Table 1. Our results revealed that, the CLogP (factor of the lipophilicity) [44] was less than 5.0, hydrogen bond acceptors between (5 and 7), hydrogen bond donors (1), this data show these compounds fulfill Lipinski’s rule. Also, the absorption percent is ranged between (~66% - 90%).
The HOMO and LUMO of a molecule play important roles in intermolecular interactions [33] , through the interaction between the HOMO of the drug with the LUMO of the receptor and vice versa. The interactions were stabilized inversely with energy gap between the interacting orbitals. Increasing HOMO energy and decreasing LUMO energy in the drug molecule lead to enhancement stabilizing interactions, and hence, binding to the receptor. Furthermore, the global and local chemical reactivity descriptors for molecules have been defined (Table 1), like softness (measures stability of molecules and chemical reactivity), hardness (reciprocal of softness), chemical potential, electronegativity (strength atom for attracting electrons to itself), electrophilicity index (measuring lowering energy due to maximal flowing electron between donor and acceptor) [45] -[51] . The results were shown in Table 1 and may explain the less toxicity and high affinity of its compounds against COX.
Acknowledgements
This study was supported by the Chemistry Department, Faculty of Science, Al-Azhar University. We are deeply thankful to Micro Analytical Center for making the IR, 1H NMR, 13C NMR and MS samples.