Horseradish Peroxidase Biosensor to Detect Zinc Ions in Aqueous Solutions ()
Enzyme based biosensors represent an alternative method to quickly detect trace metal ions through inhibition. They possess advantages such as minimum sample pretreatment, low cost and less time of analysis, and sufficient sensitivity and selectivity [6-8]. In inhibition-based enzyme biosensors, the concentration of heavy metal ions in an assayed sample is determined through either direct or indirect inhibition methods with lower detection limits. The detection principle of the enzyme-based biosensors is based on the target analyte selectively inhibiting the activity of the immobilized enzyme resulting in a decrease in voltammetric signal. A decrease in enzyme activity below normal values can serve as a standard measure which is an indicator of possible heavy metal poisoning.
In this study, horseradish peroxidase (HRP) was immobilized onto maize tassel-multiwalled composite (MTMWCNT) through adsorption to construct an inhibitor biosensor for the determination of Zn2+ ions in aqueous solution. The measurement was performed with Zn2+ inhibiting the catalytic activity of HRP enzyme to reduce H2O2. The decrease in the reduction current is expected to be proportional to the concentration of the Zn2+ ion in solution. The mode of inhibition was investigated using the Dixon and Cornish-Bowden plots.
2. Experimental
2.1. Reagents and Apparatus
All reagents were of analytical grade and were used without further purification. Horseradish peroxidase (HRP, 250 U∙mg−1), N,N-dimethylformamide (DMF), Nafion (5% ethanol solution), Multi-walled carbon nanotube (MWCNT), Zn(NO3)2 were purchased from Sigma-Aldrich (South Africa). Hydrogen peroxide (H2O2, 30% w/w) was obtained from Merck (South Africa) and solutions were freshly prepared before being used. Phosphate buffer solutions with various pH values were prepared by mixing standard stock solutions of 0.10 M Na2HPO4 and 0.10 M NaH2PO4 and adjusting the pH with 0.1 M H3PO4 or NaOH from Merck, South Africa. All solutions were prepared using Milli-Q water (resistivity ˃18 MΩcm−1). All electrochemical experiments were performed with a Bioanalytical Systems (USA) CV-50 W conventional three-electrode system. A three-electrode system was employed with an Ag/AgCl as the reference electrode, a platinum wire as the counter electrode, and the modified electrodes as the working electrodes. The phosphate buffer solution was kept in thoroughly anaerobic conditions by purging it with high-purity nitrogen for at least 15 min before and continuously during the experiments. FT-IR spectra of the free HRP, HRP/MT-MWCNT film and MT-MWCNT film were obtained using a Nicolet 380 FT-IR spectrometer (Thermo Scientific). After enzyme immobilization, the film was scraped gently from the electrode surface. The HRP/MT-MWCNT film, free HRP and MT-MWCNT film were analyzed directly using the diamond tip in the region 400 - 4000 cm−1. To avoid interferences from CO2 and water, IR chamber was flushed with N2 gas and fresh background was recorded and utilized prior to recording spectra of sample. All measurements were carried out at room temperature (25˚C ± 2˚C).
2.2. Preparation of the Maize Tassel Powder
The maize tassel (MT) powder was prepared using a reported procedure [9]. Briefly, maize tassel was plucked off the woody parts of the maize plant, thoroughly washed with water and air-dried at room temperature. The material was then milled and fractionated to obtain particles of diameter range of 50 - 100 µm which were washed twice with 0.01 M Hydrochloric acid (HCl) in order to remove any impurities that might be on the powder. The acid-washed biomass was then washed twice with high purity water prior to electrode modification.
2.3. Fabrication of HRP Biosensor
The biosensor was prepared following the steps described in our previous work [8]. Briefly, prior to modification, the GC electrode (ɸ = 4 mm) was polished to a mirror finish by use of the BASi polishing kit with 1.0, 0.3 and 0.05 μm diamond slurry in sequence, rinsed thoroughly with ultra-pure water, then ultrasonically rinsed with ethanol and ultra-pure water for 10 min in sequence, in order to remove any adsorbed substances on the electrode surface. The cleaned GC electrode was dried in air. The composite was prepared by dispersing MT-MWCNT (4:1 w/w) with the aid of ultrasonic agitation for 1 h in 10 mL of N,N-dimethylformamide (DMF) to give a 0.1 mg∙mL−1 yellow-black solution. A 10 μL drop of this dispersion was cast on to the surface of a GC electrode and after drying in air, 10 μL horseradish peroxidase (HRP) solution (10 mg∙mL−1, dissolved in 0.1 mol∙L−1 pH 7.0 phosphate buffer solution, PBS) and 0.5 μL of 0.3% Nafion to act as a binder was deposited on MT-MWCNT composite (see Figure 1).
2.4. Inhibition Studies
The determination on the decrease in the current obtained for the reduction of H2O2 by HRP using voltammetry was used as basis of evaluation. The process was carried out in a three step procedure. The biosensor response was first measured in 0.1 M PBS, (0.1 M KCl, pH 7.0) and 0.1 mM H2O2 to have steady-state current before inhibition (Ii). The electrode was then washed with the same buffer and incubated in solution containing known concentrations of Zn2+ (0.35 - 6.0 mg∙L−1). After incubating for 20 min, the modified electrode response was measured and this corresponded to steady-state current after inhibition (IF). The percentage of HRP inhibition (%IHRP) and residual enzyme activity (%REAHRP) were calculated using Equations (1) and (2) [10]:
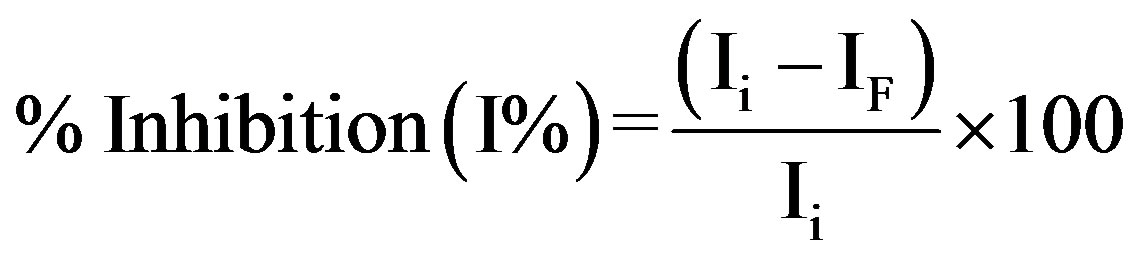 (1)
(1)

Figure 1. Schematic representation of fabrication process and inhibition process.
 (2)
(2)
3. Results and Discussion
3.1. Physical Ccharacterization
The interaction between HRP and MT-MWCNT was evaluated with the FT-IR spectra of HRP, HRP/MTMWCNT film and MT-MWCNT composite film. The shapes and positions of the amide I (1700 - 1600 cm−1) and amide II (1620 - 1500 cm−1) infrared absorbance bands of proteins provide detailed information on the secondary structure of polypeptide chain of the proteins [11,12]. Figure 2 (curve a) shows the FTIR spectrum of free HRP. The amide I band of HRP, which is caused by C=O stretching vibrations of peptide linkages, appeared at 1641 cm−1. The signal at 1516 cm−1 indicates the characteristic of amide II, which may have originated from a combination of N-H in plane bending and C-N stretching vibrations of the peptide groups. In Figure 2 (curve b) the FTIR spectrum of HRP immobilized on the surface of MT-MWCNT is shown. It can be seen in Figure 2 (curve b) that the amide I and II bands in FTIR spectrum exhibited similar shapes to that of free HRP in the FT-IR spectrum (curve a) except that the bands only shifted slightly to 1636 and 1514 cm−1, respectively. The FTIR spectrum of MT-MWCNT did not show observable peaks in the range of amides II (curve a). The FTIR results indicated that the structure of HRP remained almost unchanged in MT-MWCNT composite.
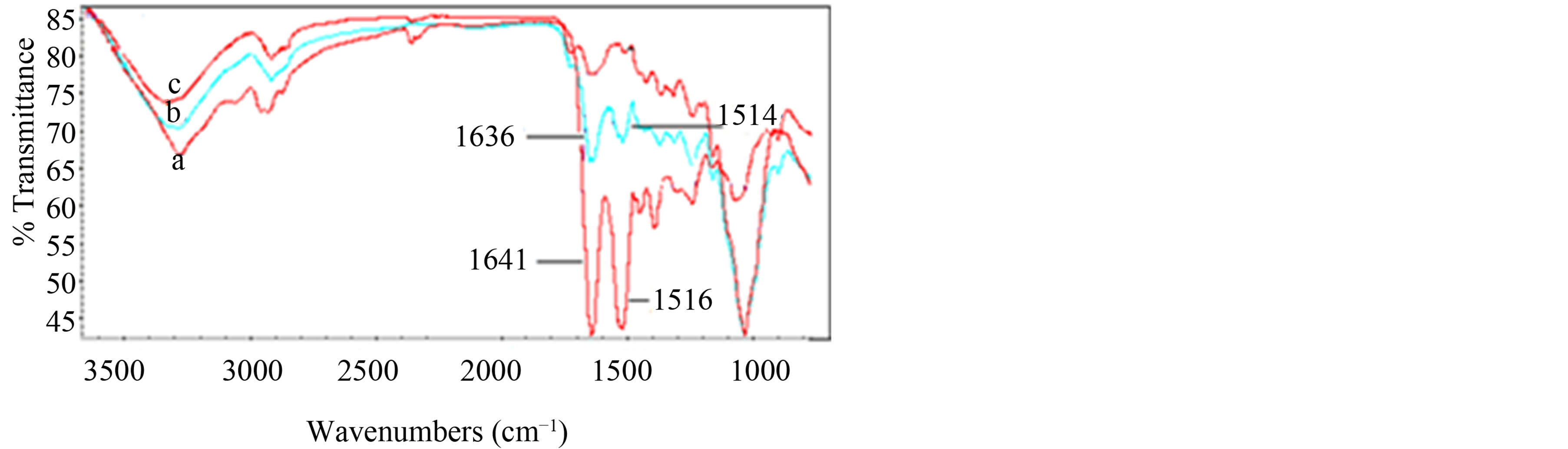
Figure 2. FT-IR spectra of the free HRP (a), HRP/MTMWCNT film (b) and MT-MWCNT film (c).
3.2. Biosensor Characterization
The enzymatically reduction of H2O2 was evaluated by using the fabricated HRP biosensor by cyclic voltammetry in 0.1 M PBS at pH 7.0. At these experimental conditions, a cathodic peak around −320 mV versus Ag/AgCl was obtained. Figure 3 shows the cyclic voltammograms of the biosensor in the absence (ab) and presence of H2O2 (0.01 - 0.5 mM) (b to f) in PBS (pH 7.0) at the scan rate of 100 mV∙s−1. An increase in cathodic peak current was observed with increase in substrate concentration.
3.3. Inhibition Studies
Typical percentage inhibition-concentration and percentage residual activity-concentration plots of the HRP/ MT-MWCNT biosensor under the optimized experimental conditions for Zn2+ are displayed in Figure 4.
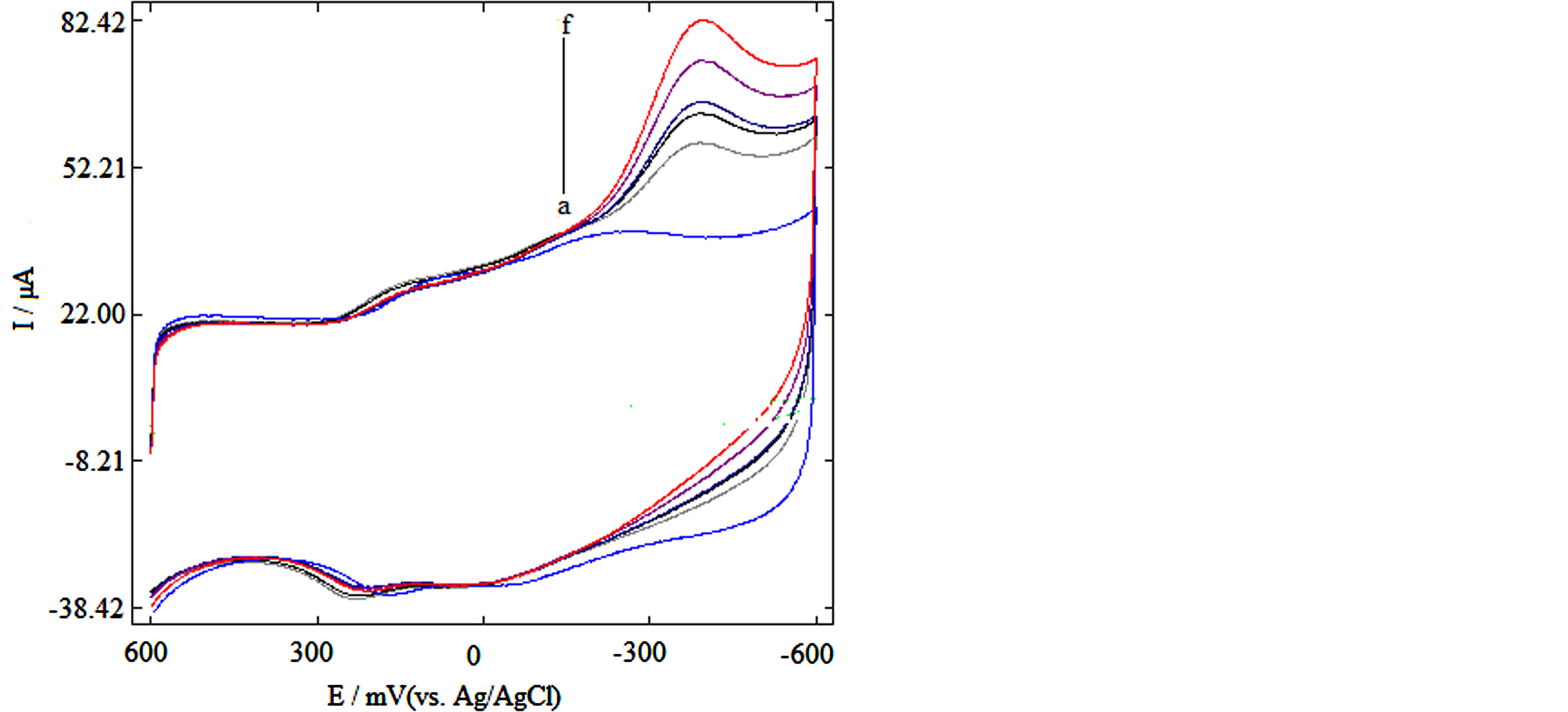
Figure 3. (A) Cyclic voltammograms of HRP biosensor in 0.1 M PBS, pH 7.0 and 0.1 M KCl (a) without substrate, (b-f) with 0.01 - 0.5 mM substrate.
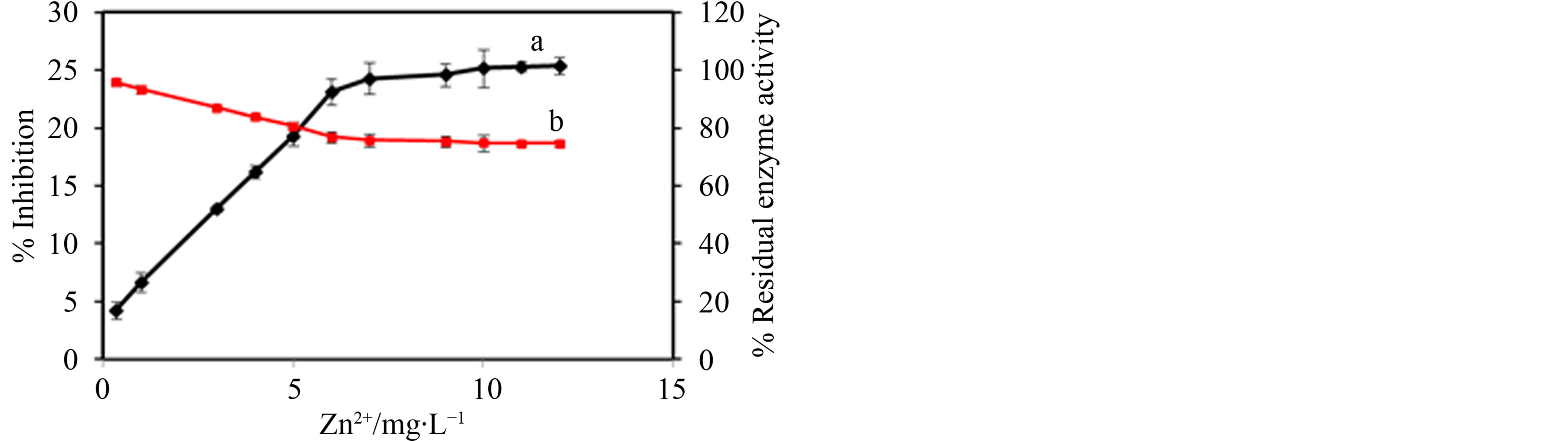
Figure 4. Dose-dependent enzyme inhibition (a) and residual enzyme activity (b) of Zn2+ towards HRP-catalyzed H2O2, Error bar = ±S.D. and n = 3.
As shown in Figure 4, this type of inhibition effect exhibited dose-dependent behavior. The percentage inhibition increased with increase in concentrations of Zn2+ ions. As can be seen in Figure 4, 25.4.0% of the activity of HRP was inhibited by 5 mg∙L−1 for Zn2+. Zn2+ had a linear range up to 12.0 mg∙L−1, and the detection limit was 7.5 μg∙L−1. The results obtained for this biosensor were comparable to those reported in literature. Using glucose oxidase immobilized in poly(neutral red) a linear range up to 2.5 mg∙L−1 and a detection limit of 9 µg∙L−1 was reported for Zn2+ [13]. In another study, Zn2+ was determined by a conductometric nitrate reductase biosensor and gave a linearity of 40 µmol∙L−1 and a detection limit of 0.5 µmol∙L−1 [14]. The residual enzyme activity decreased with increase in heavy metal ion concentration.
3.4. Investigation on the Type of Inhibition
The mode of enzymatic reversible inhibition is variable from one inhibitor to another, and may be competitive, non-competitive, and uncompetitive or mixed inhibition [15,16]. In this study, the type of inhibition shown by Zn2+ over immobilized HRP was studied using increasing concentrations of the trace metal and of the substrate, H2O2. Moreover, data modelling using Dixon plot (representation of the inverse of the enzyme activity vs. inhibitor concentration) and Cornish-Bowden plot (the ratio of substrate concentration and enzyme activity vs. inhibitor concentration) was utilized to verify the inhibition mode [17,18]. During inhibition, it should be noted that the different types of inhibition can be characterized by analyzing these two plots together. The Dixon plot by itself cannot clearly distinguish between competitive and mixed inhibition and on the other hand, the CornishBowden plot cannot always distinguish between mixed and uncompetitive inhibition. In this study, the type of inhibition shown by Zn2+ was studied using three different concentrations of H2O2 (0.05, 0.2, and 1.0 mM). Representative Dixon and Cornish-Bowden plots are shown in Figures 5A and B for Zn2+.
The pattern of inhibition shows that the Dixon plot lines are parallel (Figure 5A) hence the inhibition constant could not be determined. In the Cornish-Bowden plot, the lines intersect above the second quadrant above the x-axis, giving a value of  (1.2 mg∙L−1) (see Figure 5B) and showing that the inhibition is uncompetitive. In uncompetitive inhibition, binding occurs only to ES
(1.2 mg∙L−1) (see Figure 5B) and showing that the inhibition is uncompetitive. In uncompetitive inhibition, binding occurs only to ES

Figure 5. Dixon (A) and Cornish-Bowden (B) plots of the effect of different Zn2+ concentrations on HRP.
complexes at locations other than the catalytic site (see Figure 6). The substrate binding modifies enzyme structure, making inhibitor-binding site available [15,16,19]. In the Figure 6, E represents the HRP enzyme; S represents H2O2; ES represents Compound I containing an oxylferryl centre and porphyrin cation radical; EIS represents HRP-Zn2+-H2O2 complex; I represents the Zn2+ and P represents H2O.
3.5. Selectivity of HRP/MT-MWCNT Biosensor
Selectivity is an important parameter in the performance of an HRP/MT-MWCNT inhibition based biosensor. The addition of the following interferents; cations such as Ca2+, Mg2+, Na+, K+ and anions: F−, CN−, 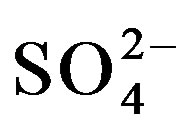 ,
, 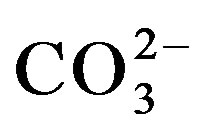 were studied by the mixed method, using the ratio of 1:2 for analyte and interferents, respectively. From the results in Table 1, the cations and anions do not cause much decrease in biosensor response, except CN− anions as also reported [20].
were studied by the mixed method, using the ratio of 1:2 for analyte and interferents, respectively. From the results in Table 1, the cations and anions do not cause much decrease in biosensor response, except CN− anions as also reported [20].


Figure 6. Mechanism for reversible, uncompetitive inhibition.

Table 1. Possible interference tested with the HRP/MTMWCNT biosensor.
3.6. Application
To demonstrate the feasibility of the fabricated enzyme inhibition biosensor for possible environmental applications, preliminary application of the biosensor was examined by determination of Zn2+, in tap water by standard addition method. The results are given in Table 2. The recoveries were in the range of 98.0% - 103.0%, which indicated the efficacy of the biosensor for practical analysis.
The validation of the HRP/MT-MWCNT biosensor measurements against the ICP-OES technique verified the suitability of biosensor for rapid analysis of trace elements in natural water standard reference material®, 1640a. The concentration of Zn2+ (3.98 µg∙L−1) in the natural water standard reference material® from National Institute of Standard and technology (NIST) were calculated from the calibration curves. The obtained results after analysis for the trace metal presented in Table 3, corroborated well with those obtained by ICP-OES, with relative error values lower than 10%.
The allowed mmaximum ccontaminant levels (MCLs) by USEPA [21] in drinking water is 1 300 µg∙L−1 for Zn2+. The World Health Organization [22] on the other hand has given the guideline values for Zn2+, in drinking water as 2 000 µg∙L−1. Based on this, it can be suggested that the HRP/MT-MWCNT biosensor could be used as a management tool for determining the quality of water for the presence of Zn2+ ions.
3.7. Stability, Repeatability and Reproducibility
The stability of the biosensor was first examined in the presence of 0.1 mM H2O2 concentration in 0.1 M PBS (pH 7.0). For the same metal concentration, it was observed that after 10 successive series of measurements, the biosensor lost about 30% of the initial sensitivity. In studying the long-term stability, the HRP/MT-MWCNT biosensor was stored in 0.1 M PBS at 4˚C for 18 days and the biosensor response was tested on different days after incubation in the inhibitor. The biosensor did not

Table 2. Recovery test for Zn2+ in tap water.

Table 3. Evaluation of the HRP/MT-MWCNT biosensor.
show a bigger decrease of its initial response for 0.1 mM H2O2 after incubation in standard Zn2+ ion solution for the different days studied. The repeatability of the HRP/ MT-MWCNT biosensor was investigated for fixed Zn2+ ion concentrations. Relative standard deviations (RSD) of 5.3% were obtained for Zn2+. Five modified biosensors were made independently and were investigated for the determination of the same concentrations of Cu2+. The modified biosensors showed a relative standard deviation (RSD) of Zn2+ (4.8%).
4. Conclusion
The use of an HRP/MT-MWCNT biosensor for the determination of Zn2+ ions through inhibition studies has been demonstrated. It was deduced that HRP was inhibited by Zn2+ ion. The highest inhibition result obtained for the HRP/MT-MWCNT biosensor was 25.4%. The metal ion was measured with a detection limit of 7.5 µg∙L−1. By modelling the data and using the CornishBowden together with Dixon plots, the inhibition was determined to be reversible and uncompetitive. The proposed biosensor does not require any complicated immobilization procedure for the construction and has been shown to detect low concentration in samples.
Acknowledgements
The author would like to acknowledge Midlands State University.