Fluid Flow and Sub-Bactericidal Release of Silver from Organic Nanocomposite Coatings Enhance ica Operon Expression in Staphylococcus epidermidis ()
1. Introduction
Along with the implantation of artificial organs and medical devices, the use of synthetic materials has become an indispensable part in almost all fields in medicine. Poly (ethylene terepthalate) (PET) is used in certain medical implants such as artificial heart valve sewing rings and artificial blood vessels due to its good mechanical properties and relatively high biocompatibility [1]. However, as is the case of most biomaterials, its long-term use is impeded by infections [2,3]. While a variety of microorganisms are involved in medical device-associated infections, coagulase-negative staphylococci, specifically Staphylococcus epidermidis, has been identified as a predominant cause of infection in the presence of a medical device [4], due to its ability to adhere to surfaces, followed by the production of slime, and the formation of biofilm [5,6]. In the case of S. epidermidis the accumulative phase of biofilm formation mainly depends on polysaccharide intercellular adhesin (PIA) synthesis that is encoded by the icaADBC operon [7,8].
Recent publications have outlined the necessity to develop a strategy to reduce bacterial adhesion to aortic grafts [9,10]. The main goals of management are the removal of the infected graft material and the re-establishment of vascular continuity [9,11]. However, these procedures are associated with a high mortality and re-infection rate. Moreover, conservative management comprising long-term intravenous antibiotics has been reported [12]. Although the loading of materials with antibiotics seems to be an effective method to reduce biofilm formation, the ongoing release of antibiotics promotes the development of resistant strains and the risks for the spread of such resistance, following the biomaterial prophylactic and therapeutic clinical use [13].
An alternative to antibiotics for reducing bacterial viability and adhesion on medical devices is to focus on materials that release antimicrobial agents such as Ag [14]. It has been suggested that impregnation of Ag into a coating can be more effective than direct surface coating alone, since surface Ag can be readily deactivated by protein anions, while Ag release cannot be controlled in the case of Ag coatings [15,16]. Polymers that release Ag in the oxidised form have shown strong antibacterial activity and would act as reservoirs of Ag and be capable of releasing Ag for extended periods [17].
In this direction the present study investigated the effectiveness of Ag-containing organic coatings (Ag: CxHyOz) plasma-deposited on polyethylene terephthalate (PET), which contains oxygen functionalities that can be involved in the mechanism of Ag release, against initialbacterial adhesion and biofilm formation and how this is related to icaA gene expression.
Assessment of icaA DBC operon genes’ expression has become crucial to the understanding of the pathogenesis of S. epidermidis infections [18,19], and reverse transcription (RT) followed by polymerase chain reaction (RT-PCR) represents a powerful tool for gene expression studies through the detection and quantification of mRNA. Quantification is realized by the relative RTPCR by the determination of the expression level of the target gene versus a housekeeping gene [20]. The icaA gene product is a transmembrane protein with homology to N-acetyl-glucosaminyltransferase [21]. The icaA gene was therefore selected to be tested as marker of ica operon activity, because it encodes the first component of biofilm formation.
Although there is evidence that Ag interferes with the bacteria-bacteria interactions [22], according to our knowledge, there is no publication so far relating the effect of Ag on icaA expression.
Moreover, since the process of bacterial adhesion to indwelling medical devices is associated in most cases with flow of body fluids [23], in this work bacterial adhesion to the various substrates was examined under two shear rates 50 and 2000 s−1, which corresponded to the physiological shear rates for stable laminar flow in blood vessels, and the effect of shear rate on icaA expression was investigated as well.
2. Materials and Methods
2.1. CxHyOz and Ag:CxHyOz Coatings Deposition
Organic coatings containing Ag were deposited on PET substrates through a combined strategy in which a plasma enhanced chemical vapour deposition process is simultaneous with a sputtering ones. A flow of Diethylene glycol dimethyl ether vapours of 0.25 sccm mixed with 20 sccm of argon was used as gas feed. An asymmetrical, parallel plate plasma reactor described in detail elsewhere was used for the plasma depositions [14]. Briefly, the reactor is equipped with a 7 cm dia. RF electrode and a ground one of 18 cm separated each other by a gap of 6 cm. Due to the smaller area of the cathode, under proper experimental conditions (high RF power, low pressure, low DEGDME/Ar flow ratio), a bias-induced ion bombardment results at this electrode, which prevents the deposition of any coating, and induces the sputtering of silver atoms, as evidenced by Optical Emission Spectroscopy [24,25]. Substrates were positioned at the large, ground electrode. The chemical composition of Ag:CxHyOz films (C/O/H composition, Ag-content), as well as the dimensions of the Ag clusters can be tuned by adjusting the deposition parameters. A pressure of 50 mTorr and a power of 60 W were used for the plasma depositions.
In order to investigate if the nanocomposite containing coating Ag or the organic one is responsible for the antibacterial character of the material, plasma deposition of the organic coating was realized under the same experimental conditions by replacing the silver target with a stainless steel one.
2.2. Physicochemical Characterization of the Coatings
The plasma deposited coatings were analyzed by means of physicochemical characterizations performed using X-ray Photoelectron Spectroscopy (XPS), Water Contact Angle (WCA) measurements, Atomic Force Microscopy (AFM) and Ag release measurements. The aim of such analysis was to assess if the chemical composition of the organic matrix (i.e. CxHyOz) was the same with or without the presence of Ag embedded in the coating, to assess if the chemical/morphological changes derived from the inclusion of Ag in the coating can dramatically affect the wettability of the analyzed samples, to investigate if there was any change in the surface morphology that can be attributed to the presence of Ag clusters included in the organic layer during plasma process and to evaluate the amount of Ag that was released from the substrate within the first 24 h.
2.2.1. X-Ray Photoelectron Spectroscopy
(XPS) measurements were performed with a Theta Probe Thermo VG Scientific instrument (base pressure 1 × 10−9 mbar) equipped with a monochromatic AlKα radiation (hν: 1486.6 eV) operating at 300 W with a spot size of 400 µm. The analysis was carried out by an angle resolved mode acquiring simultaneously information from different take-off angles (T.O.A.s) ranging from 59.5˚ to 14.5˚ with a conical angle of acceptance of 33˚, by using a two-dimensional detector that collects signal intensities in both photoelectron energy and photoemission angle. The two-dimensional detector was placed at the output plane which has the photoelectron energy dispersed in one direction and the angular distribution dispersed in the other direction. Samples were neutralized for the electrostatic charging by means of a flood gun (Mod. 822-06 FG) operating at 400 µA emission current, 40 V extraction voltage at 2 × 10−7 mbar to correct differential or non-uniform charging. The high resolution spectra were shifted to their correct position by taking C1s spectrum centred at 285.0 eV as reference [26].
2.2.2. Water Contact Angle Measurements
A Ramè-Hart-NRL mod. 100 was used to measure dynamic water contact angle (WCA) values with double distilled water. Advancing WCA (θadv) was measured by increasing the volume of the water drop in 2 µl steps, until the WCA remained constant. Receding WCA (θrec) was measured by decreasing the volume of the drop until the WCA value remained constant and the solid/liquid interface started to decrease. Five samples from each group were utilized for WCA measurements. For each sample, five readings of θadv and θrec were measured at different regions and averaged.
2.2.3. Atomic Force Microscopy (AFM)
Topographic AFM images were collected in tapping mode by using a PSIA XE-100 SPM System operating in air and at room temperature. A high frequency silicon cantilever for non-contact/tapping mode purchased from NanoWorld was used. A silicon SPM sensor for noncontact AFM (Park Systems) having a spring constant of 42 N·m−1 and a resonance frequency of 330 kHz was used. Micrographs were collected on six areas of each sample, with a scan size area of 5 × 5 µm2, by sampling the surface at a scan rate within 1.0 - 0.5 Hz and a resolution of 256 × 256 pixels. Topography AFM images were processed by using the XEI software to obtain statistical data of the surface root-mean-squared (RMS) roughness values of the films and mean heights of sample features.
2.2.4. Silver Release
The amount of Ag release was evaluated by Inductively Coupled Plasma Mass Spectrometry (ICP-MS) analysis which was performed by means of aTJA-IRIS-Advantage spectrometer (laboratory CHIMIE s.r.l. Triggiano, Bari) with echelle optics and Charge Injection Device (CID) semiconductor detector, axial and radial viewing and a wavelength range 170 - 900 nm. Plasma coated samples were soaked in 50ml of double distilled water at 37˚C for 24 hours and the liquid was analyzed by the ICP-MS.
2.3. Bacterial Culture Conditions
The bacterial strain used in this study was the ica-positive slime producing reference S. epidermidis ATCC 35984. Bacteria that were in the mid-exponential growth phase, after growth in Brain-Heart Infusion Broth (BHIB, Difco Laboratories, Detroit, USA), were harvested and resuspended in 0.9% NaCl at a concentration of 3 × 109 Colony Forming Units (CFUs)/ml [27].
2.4. Dynamic Bacterial Adhesion Assays
To evaluate the bacterial adhesion under flow conditions and investigate the combined effect of flow and surface chemistry on ica gene expression, the parallel plate flow chamber (PPFC) described by Stavridi et al. [28], was used. The pump was programmed to travel the pistons back and forth every 60 s. The shear rate (S) was calculated by the following formula:
 (1)
(1)
where Q is the flow rate, W (width of the chamber) = 0.015 m and h (height of the chamber) = 0.35 × 10−3 m.
All experiments were carried out at 37˚C by placing the experimental setup inside a thermostated box (INFORS HT, Bottmingen, Switzerland), as described by Foka et al., [27]. Two shear rates were used: 50 and 2000 s−1 and bacterial adhesion and gene expression were examined two hours post adhesion.
2.5. Examination and Quantification of Bacterial Adhesion and Biofilm Formation
2.5.1. Colony Forming Units Counting Method
After the adhesion experiments, adherent bacteria were detached by immersing each sample in trypsin (SigmaAldrich) at 37˚C for 5 min and using a cell scrapper (Sigma-Aldrich). Trypsin was inactivated with Fetal Bovine Serum (FBS, Sigma-Aldrich). Then 10-fold serial dilutions of the detached adherent bacteria were inoculated onto Trypticase Soy Agar (TSA, Difco Laboratories, Detroit, USA) plates, and the numbers of adherent bacterial colonies were counted after 18 h of incubation at 37˚C.
2.5.2. Scanning Electron Microscopy
In order to be examined with Scanning Electron Microscope (SEM)(JEOL-JSM 6300, Hertfordshire, UK), each sample was fixed, dehydrated and sputter coated with gold. Images were processed using the Image Pro Plus Analysis Software (Media Cybernetics), in order to quantify the percentage of surface area covered by bacteria.
2.5.3. icaA Gene Expression Study
After the adhesion experiments, adherent bacteria were detached by trypsin (Sigma-Aldrich) and collected by centrifugation, as described by Foka et al., [27]. The bacterial pellets, kept at −20˚C, were used for RNA isolation, within a week after collection using the Trizol method (Invitrogen, Carlsbad, USA), while the samples were examined in sets of four so that their processing was well controlled and consistent [27]. Genomic DNA contamination was tested by PCR with specific primers, using the appropriate reaction mix composition and thermal conditions for icaA gene [29] and a 207 bp part of the region V in the 23S rDNA gene [27]. Complementary DNA (cDNA) synthesis was carried out using 0.5 ng total RNA, 50 ng random hexamers primers and the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen).
Real Time PCR for icaA gene and the 207 bp part of the region V in the 23S rDNA gene were carried out with the RotorGene device (RG-3000, Corbett Research, Sydney, Australia). The same primers used for the conventional PCRs were applied [27,30]. Each run included one ica-negative and a non template control as negative controls. The specificity of the PCR products was confirmed by analysis of the dissociation curve. The results were evaluated using the Rotor-Gene Analysis Software 6.0. The efficiency of reactions was calculated from the slope according to the equation E = 10−1/slope [20].
Relative RT-PCR constituted the rate of the expression levels of icaA comparing the absolute values towards those of the reference gene (23S rDNA) [27,31].
3. Statistical Analysis
The effects of the surface chemistry and flow conditions on bacterial adhesion, biofilm formation, and icaA geneexpression were statistically analyzed using the SPSS package for windows. One way analysis of variance (ANOVA) was performed using the Scheffe significant difference test. Moreover, regression analysis and correlation coefficients (R2) were obtained using SPSS. Correlations were taken as significant for p < 0.01.
4. Results
4.1. X-Ray Photoelectron Spectroscopy
Figure 1 shows the low resolution XPS spectra acquired on CxHyOz coatings deposited in the same experimental conditions but with (i.e. Ag:CxHyOz) or without (i.e. CxHyOz) the presence of Ag embedded inside the organic matrix. When a stainless steel target was used instead of an Ag one a presence of a coating containing no other elements than carbon and oxygen is deposited as confirmed by the XPS spectrum acquired on CxHyOz sample (Figure 1). By replacing the stainless steel target with a Ag one, a coating containing Ag was obtained as shown by the presence of the characteristic Ag3d, Ag3p, Ag4p, Ag4s and Ag4d peaks in the low resolution spectrum (see Ag:CxHyOz spectrum in Figure 1).
The best fitting of the XPS C1s spectrum acquired on Ag:CxHyOz coatings (Figure 2(a)) shows the presence

Figure 1. Low resolution XPS spectra acquired on CxHyOz and Ag:CxHyOz samples.
of different oxygen containing functionalities. The percentage of hydroxyl/ether groups attesting for the retention of the chemical structure of the precursor (i.e. DEGDME) in the coating was very low. Table 1 presents the elemental percentages of C, O and Ag of the CxHyOz and Ag:CxHyOz coatings. The CxHyOz coatings presented an O/C ratio 0.36, while the O/C for DEGDME is 0.5. These results indicate that the experimental conditions (i.e. high power low amount of precursor flow and low pressure) used for the plasma processes contributed to a certain fragmentation of the precursor during the discharge and a certain ion bombardment of the organic deposited film during the process that produces a high cross linking of the deposited film (i.e. high hydrocarbon content). In the case of the Ag:CxHyOz coating the O/C ratio was 0.29 and the percentage of Ag into the nacocomposite reached almost 20%.
To assess if the organic matrix is the same with or without the presence of Ag clusters, an overlapping of high resolution XPS C1s spectra is reported in Figure 2(b). It is possible to see that the C1s spectra are almost identical confirming that the presence of Ag did not change the chemical characteristics of the organic layer and that the coating CxHyOz can be used as reference of Ag:CxHyOz ones during antibacterial tests to disentangle the effect of the organic matrix to that one of silver.
4.2. Water Contact Angle Measurements
The hydrophilic character of plasma deposited coatings dramatically changed when Ag was introduced. As shown in Table 1 the presence of Ag clusters in the coating increased the advancing water contact angle (qadv) from 68 ± 3˚ in the case of the CxHyOz coating to 104 ± 7˚ in the case of the Ag:CxHyOz one. Contemporary a very evident decrease of receding water contact angle (qrec, Table 1) was observed for the Ag:CxHyOz samples. These findings show that a very important hysteresis was associated to the presence of Ag clusters. Hysteresis is defined as the difference between the maximum and the minimum WCA values measured in a dynamic mode onto a surface, i.e. the maximum angle while the droplet is increasing its volume (θadv) and the minimum just be fore the reduction of the contact area with the solid during the volume decreasing (θrec). The hysteresis is originated by the presence of heterogeneities (in topography and/or chemical composition) which induce fluctuations in surface tension [32]. Since hysteresis originates from defects, the high hysteresis registered on Ag:CxHyOz samples can be attributed to certain surface roughness other than chemical heterogeneity.
4.3. Atomic Force Microscopy (AFM)
Atomic Force Microscopy (AFM) was employed to investigate the morphology of the Ag:CxHyOz samples and further explore the hysteresis observed in the WCA measurements. Figure 3 reports the comparison of the topography of the CxHyOz coating with that of the Ag:CxHyOz one. The image shows that the CxHyOZ coating appeared quite smooth with aroot mean square (RMS) roughness value of 0.2 ± 0.1 nm. Implementation of Ag into the organic coating significantly changed the morphology of the coating. Indeed, the RMS value increased up to ca. 7.43 ± 0.37 nm and the surface was characterized by a dense and highly interconnected network of round-shape features having a mean height of 71.3 ± 4.5 nm accounted for by Ag clusters.
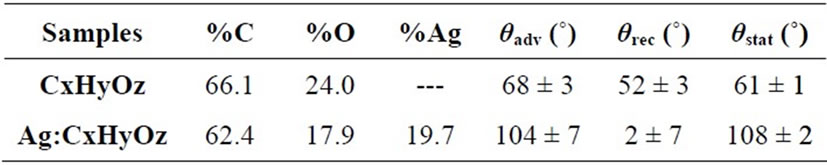
Table 1. XPS chemical composition of the plasma deposited coatings and results of water contact angle measurements.
 (a) (b)
(a) (b)
Figure 2. (a) XPS C1s spectrum acquired on Ag:CxHyOz sample and best fitted in four components centred at 285.0 ± 0.2 eV (alkyl, C-C; C-H), 286.5 ± 0.2 eV (hydroxyl/ether, C-OH/COR), 287.8 ± 0.2 eV (carbonyl/aldehyde or Hemiacetal/Hemiketal, >C=O/O-C-O), 289.2 ± 0.2 eV (carboxyl/ester, COOH/COOR); (b) overlapping of C1s spectra acquired on CxHyOz and Ag:CxHyOz samples.

Figure 3. 2DAFM topography images of bare a) CxHyOz and b) Ag:CxHyOz films. In panel c) cross sectional line profile taken along the red line of panel B.
4.4. Silver Release
ICP-MS characterization of plasma modified materials show a release of silver of 200 ± 20 ppm/cm2 in the first 24 hrs.
5. Bacterial Adhesion and Biofilm Formation
5.1. Colony Forming Units Counting Method
Figure 4 shows the combined effect of surface chemistry and shear rate on bacterial adhesion (number of adherent bacteria/cm2), as this was quantified by the Colony Forming Unit (CFU) method. Bacteria adhered significantly more to the CxHyOz coated PET in comparison to the Ag:CxHyOz coated one(p < 0.01). A decrease in the number of adherent bacteria, for both materials, was observed when the shear rate increased from 50 s−1 to 2000 s−1. This decrease was significantly different (p < 0.01), for all possible combinations. The lowest number of attached bacteria was calculated under the higher shear rate onto the Ag:CxHyOz coated PET.