Phytotoxicity of Chromium on Germination, Growth and Biochemical Attributes of Hibiscus esculentus L. ()
1. Introduction
Heavy metals are the most imperative component of the environment that frequently accumulate in the soil due to unplanned municipal waste disposal, mining, use of extensive pesticides and chemical fertilizers [1,2]. Hyper accumulation of heavy metals may lead to toxic effect in human, animals, plants and other microorganisms and create a serious threat to biota and the environment [3].
Chromium is an environmental pollutant that ranks seventh in abundance within the earth crust [4]. Naturally occurring chromium in soil ranges from 10 to 50 mg·kg−1 [1]. The chemistry of chromium is very complex. Its solubility, mobility and bioavailability in soil strongly depend on the various oxidation states from 0 to + 6. Depending on its oxidation state and concentration, chromium acts as a toxic or essential element for living organism. The two most common species of chromium are Cr(III) and Cr(VI) available in anionic form as chromate, dichromate and hydro chromate ions. Cr(III) is essential for animal and human at low concentration. Chromium is extremely stable in soil, but usually well immobilized on iron and manganese oxides and hydroxides or complexes to organic matter. The toxicity of Cr(VI) strongly depends on its concentration in the soil and its uptake mechanism [5]. The sources of chromium in environment are both natural and anthropogenic. Chromium is used on a large scale in many industries, including metallurgical, electroplating, production of paints and pigments, tanning, wood preservation, chromium chemicals production, pulp and paper production [6]. The leather industry is the major cause of high influx of chromium to the biosphere, accounting for 40% of the total industrial use [7]. Sewage and fertilizers are also the main sources of chromium [8]. These anthropogenic activities may lead to the widespread contamination in the environment [9,10].
A high concentration of chromium was found to be harmful for plant life, reducing the protein contents, inhibiting the enzyme activity, and causing chlorosis and necrosis [11]. The chromium concentration in plant adversely affects several morphological and biochemical parameters [12]. Chromium toxicity interferes with several metabolic processes in plant, causing reduced seed germination or early seedling growth [13], biomass, photosynthetic impairing [14,15]. Phytotoxicity of chromium is considered inhibitory for plant growth. Its presence in surplus amount inside the plant can cause stunted growth [16-18]. The presence of chromium in soil disturbs the pattern of nutrient uptake in plant because of nutrient metal interaction [19,20].
Phytotoxic effects of different chromium concentration on seed germination and seedling growth in various vegetable crops, Daucus carrota (L.), Raphanus sativus (L.), Beta vulgaris (L.), Lycopersium esculentum (L.) and Solanum melongena (L.), Vigna radiata (L.), Vigna angularis (L.), Lablab purpureus (L.), Lathyrus ordoratus (L.), Triticum aestivum (L.) were reported [21-23].
The present study was conducted to investigate the phytotoxic effects of different chromium concentration on Hibiscus esculentus (L.) to illustrate the potential of this species toward chromium metal stress.
2. Material and Methods
2.1. Seed Material
Certified seeds of Hibiscus esculentus (L.) were procured from Komal Seed Production, Tando Allahyar. Seeds of uniform size, color and weight were selected for experimental work.
2.2. Soil Collection and Pot Preparation
For pot experiment soil samples were collected from riverbed of Indus, Jamshoro. The soil (2 part sand and 1 part clay) was air dried for 2 - 3 days. The pots were then filled with 5 kg air dried soil. The physicochemical properties of soil are given in Table 1.
2.3. Chemicals and Stock Solution Preparation
All chemicals used were of analytical reagent grade; an-
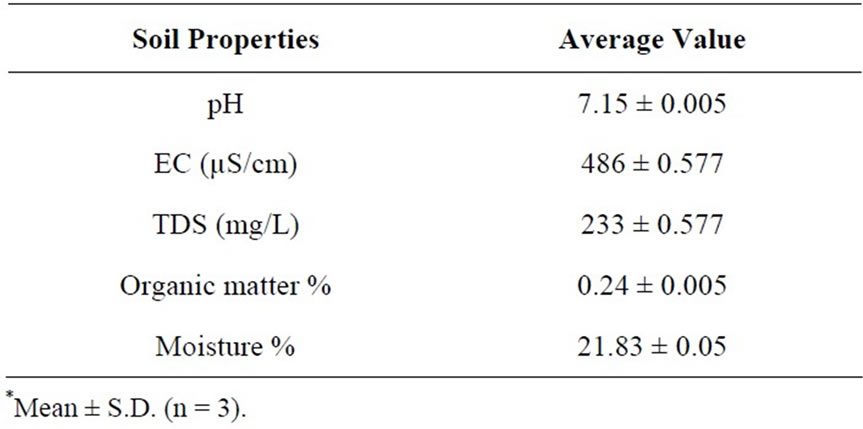
Table 1. Physico-chemical properties of initial soil samples*.
hydrous potassium dichromate were procured from Fluka (Sigma-Aldrich, Madrid, Spain) and used without any further purification. Stock solution of chromium was prepared by dissolving 2.28 g of potassium dichromate in 1000 ml of deionized water. The stock solution was then appropriately diluted to get the test solution of desired chromium concentration.
2.4. Experimental Design
A pot culture experiment was conducted at Green House of Institute of Plant Sciences, University of Sindh, Jamshoro. The experiment was arranged in complete randomized block design with three replicates for each treatment. To initiate the experiment under controlled condition, air dried soil artificially polluted with different concentration of potassium dichromate solution i.e. 0.5, 2.5, 5, 10, 25, 50 and 100 mg·kg−1 respectively along with an untreated control. Chromium solution was uniformly mixed with air dried soil and kept for 2 weeks to stabilize (Figure 1).
2.5. Seed Sterilization and Sowing
Seed surface were sterilized with 0.1% mercuric chloride solution and then rinsed with deionized water [24]. Fifteen seeds were sown with uniform distance in each pot.
2.6. Estimation of Germination and Growth Measurements
The seed germination was noted for every 24 hour until the germination percentage was constant. For the evaluation of seedling growth 10 germinated seedlings of similar morphology were allowed to grow with concentration of 0.5, 2.5, 5, 10, 25, 50 and 100 mg·kg−1 chromium in soil, irrigated on alternate day with distilled water. The

Figure 1. Pot experiment represents the phytotoxic effect of different chromium level in Hibiscus esculentus (L.) after 30 days: (a) Control (b) 0.5 mg·kg−1 (c) 2.5 mg·kg−1 (d) 5 mg·kg−1 (e) 10 mg·kg−1 (f) 25 mg·kg−1 (g) 50 mg·kg−1 (h) 100 mg·kg−1.
seedlings were harvested carefully after 30 days, washed with distilled water to remove soil particles and analyze for growth and various biochemical attributes. Growth attributes were studied in terms of root and shoot length (cm), root and shoot fresh weight (g), root and shoot dry weight (g). Seedlings were collected and cut at root-shoot junction and the length of their root and shoot were measured with a metric scale and expressed in centimeters [25]. The fresh weight of root and shoot samples were recorded on an analytical balance and expressed in gram per plant [26]. Later, plant parts were dried in an oven at 60˚C for 24 hour to get constant dry weight for root and shoot.
2.6.1. Germination Percentage
The germination percentage is the proportion, expressed as percentage of germinated seeds to the total number of viable seeds that were tested by following formula: [27,28].
%G = (Number of germinated seeds/Total number of planted seeds) × 100(1)
2.6.2. Seedling Vigor Index
Seedling vigor index are those properties of the seed which determine the level of activity and performance of the seed during germination and seedling emergence. It is a single measurable property like germination describing several characteristics associated with various aspects of the performance of seed. Seedling vigor index is calculated by following formula: [29,30]
SVI = Germination percentage × Seedling length (2)
2.6.3. Stress Tolerance Index
Stress tolerance index is a useful tool for determining the high yield and stress tolerance potential of genotypes. Stress tolerance indices for different growth parameters were calculated using following formulae [31]:
RLSTI = (Root length of stress plant/Root length of control plant) × 100(3)
SLSTI = (Shoot length of stress plant/Shoot length of control plant) × 100(4)
RFSTI = (Root fresh weight of stress plant/Root fresh weight of control plant) × 100&(5)
SFSTI = (Shoot fresh weight of stress plant/Shoot fresh weight of control plant) × 100(6)
RDSTI = (Root dry weight of stress plant/Root dry weight of control plant) × 100(7)
SDSTI = (Shoot dry weight of stress plant/Shoot dry weight of control plant) × 100(8)
2.7. Estimation of Biochemical Attributes
Biochemical attributes were studied in term of photosynthetic pigments. The chlorophyll-a, chlorophyll-b and total chlorophyll (a + b) were determined spectro-photometrically. Leaves were cut into small pieces, mixed thoroughly and 0.25 g of leaves was taken into a mortar to grind them finely by pestle with 25 ml of 80% acetone for 5 minutes. The homogenate was filtered through filter paper (Whatman® No.42) and was made a volume of 25 ml with 80% acetone.
Extract Monitoring by Spectrophotometer
After the extraction, chlorophyll contents were monitored by UV-Vis spectrophotometer (Biochrom Libra S22). The optical density/absorbance of each solution was measured at 663 and 645 nm against 80% acetone blank in 1 cm quartz cuvette at room temperature. The Arnon’s equation was used to calculate the amount of chlorophyll-a, chlorophyll-b and total chlorophyll (a + b) [32]:
Chl a (mg·g−1) = [(12.7 × A663) − (2.69 × A645)] × ml acetone/mg leaf tissue(9)
Chl b (mg·g−1) = [(22.9 × A645) − (4.68 × A663)] × ml acetone/mg leaf tissue(10)
Total Chl = Chl a + Chl b(11)
3. Statistical Analysis
Data were statistically analyzed using one-way ANOVA on PASW® Statistics 18 (SPSS Inc., Chicago, IL, USA). The results are presented as means ± S.E. (standard errors) and data from the different treatments and control were compared by Duncan’s multiple-range test at p < 0.05.
4. Results and Discussion
4.1. Effect of Chromium Toxicity on Seed Germination
The result of present study reveals that higher chromium concentration adversely influence the germination process of Hibiscus esculentus seeds (Figure 2). Chromium treatment at 0.5, 2.5, 5, 10 and 25 mg·kg−1 has no significant (p > 0.05) effect on seed germination. However, germination percentage was significantly (p < 0.05) affected at 50 and 100 mg·kg−1 by 42.22% and 48.88%
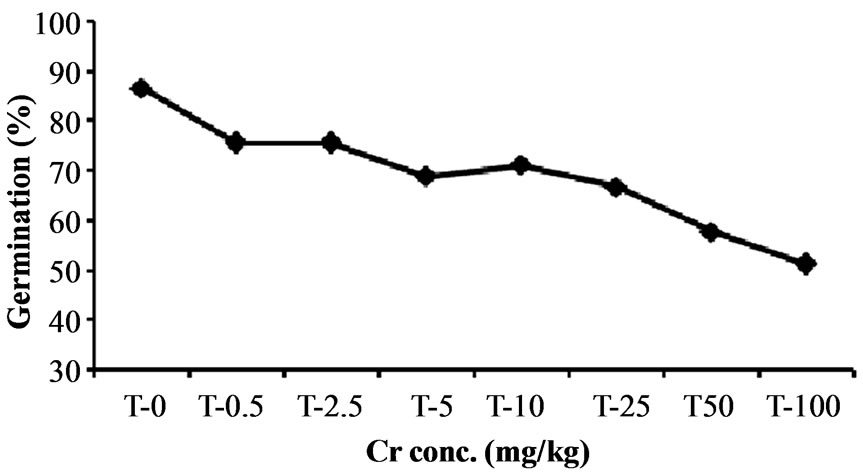
Figure 2. Effect of chromium on seed germination in Hibiscus esculentus (L.).
reduction in germination (Table 2), indicating that higher level of chromium produced toxic effect in seed germination. Similar results were reported by Zayed and Terry [15]. Increasing concentration of heavy metal significantly reduce the strength of germination as compare to the lowest concentration of heavy metal which have the least harmful influence on the germination [33].
4.2. Effect of Chromium Toxicity on Seedling Vigor Index
The increased chromium level adversely influence the seedling vigor index of Hibiscus esculentus shown in Figure 3.
Seedling vigor index of Hibiscus esculentus gradually decrease with increase chromium concentration at 0.5, 2.5, 5, 10, 25 and 50 mg·kg−1 (Table 2). Various treatment of chromium were found to be toxic at sub optimal (0.5 mg·kg−1 and 2.5 mg·kg−1) to above optimal (5 - 100

Table 2. Effects of different chromium concentration on germination and seedling vigor index in Hibiscus esculents (L.)
mg·kg−1) concentrations. The average seedling vigor index of Hibiscus esculentus reduce from 1672.66, 1385.18, 1264.29, 1155.03, 941.03, 688.88 and 333.18 respectively due to increase chromium level.
Similar results were reported by Ganesh et al. [34] that there was a reduction in vigor index in four genotypes of soybean at 5 - 200 mg·L−1 concentration of chromium, with respect to control application.
4.3. Chromium Toxicity on Root and Shoot Elongation
The results after 30th day exposure of chromium to Hibiscus esculentus, shows considerable reduction in root and shoot elongation. The length of Hibiscus esculentus was adversely affected due to chromium (Table 3). The average root length 8.66 ± 0.33, 8.23 ± 0.12, 8.10 ± 0.10 was not significantly (p > 0.05) affected at 0.5, 2.5 and 5 mg·kg−1 respectively. Although root elongation was significantly (p < 0.05) reduce at 10, 25 and 50 mg·kg−1 by 6.40 ± 1.13, 5.23 ± 0.99 and 2.43 ± 0.06 respectively. Similarly, the average shoot length of Hibiscus esculentus 9.66 ± 0.66, 8.50 ± 1.32, 8.66 ± 0.44 was not significantly affected (p > 0.05) at 0.5, 2.5 and 5 mg·kg−1 respectively. However, increase in chromium 10, 25, and
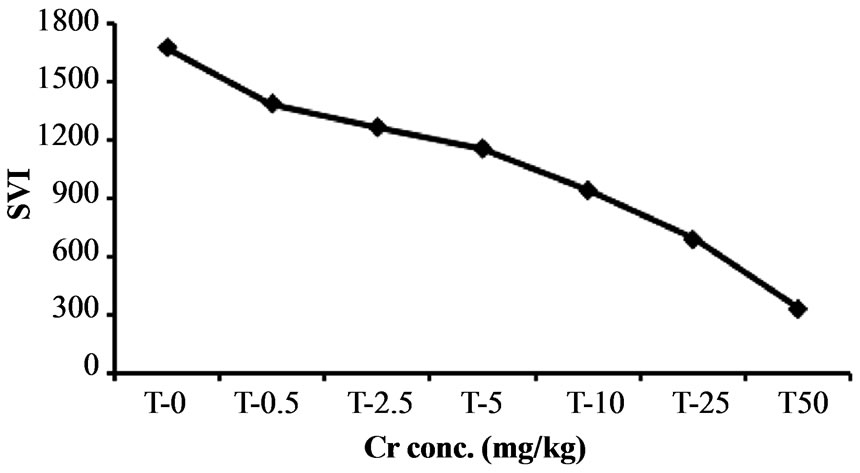
Figure 3. Effect of chromium on seedling vigor index in Hibiscus esculentus (L.).

Table 3. Effects of different chromium concentration on seedling growth of Hibiscus esculentus (L.).
50 mg·kg−1 significantly (p < 0.05) reduced shoot length with respect to control plant by 6.83 ± 0.41, 5.10 ± 1.45 and 3.33 ± 0.16 respectively. No data were found in 100 mg·kg−1 soil treatment because all plants were died at seedling stage, indicating that higher level of chromium significantly (p < 0.05) affected root and shoot elongation in Hibiscus esculentus. Several author reported that the inhibition of root length caused by heavy metals may be due to metal interference with cell division, together with inducement of chromosomal aberrations and irregular mitosis [35,36], which can be effected on seedling growth. Samantaray et al. [37] in a study by means of chromite mine pollute soil in five mung bean cultivars, noted that root growth was significantly affected 28th days after root emergence as seedling are more sensitive than seed germination for measurement of the toxic effect of chromium pollution.
4.4. Effect of Chromium Toxicity on Root and Shoot Fresh Weight
The root and shoot fresh weight of Hibiscus esculentus were severely affected due to increase chromium concentration in soil (Table 3). Result shows that chromium treatment at 0.5, 2.5, 5, 10 and 25 mg·kg−1 was not significantly (p > 0.05) affect root fresh weight by 0.19 ± 0.02, 0.19 ± 0.03, 0.16 ± 0.03, 0.16 ± 0.03, 0.13 ± 0.00 respectively. However, the root fresh weight of Hibiscus esculentus was significantly (p < 0.05) affected at 50 mg·kg−1 by 0.11 ± 0.01. Similarly, at 0.5, 2.5, 5 and 10 mg·kg−1 the shoot fresh weight of Hibiscus esculentus was not significantly (p > 0.05) affected by 0.80 ± 0.16, 0.75 ± 0.14, 0.70 ± 0.09 respectively. Although, the shoot fresh weight of Hibiscus esculentus was significantly (p < 0.05) decrease at 10, 25 and 50 mg·kg−1 by 0.39 ± 0.00, 0.31 ± 0.13, 0.14 ± 0.01 respectively. Similar results were reported by Fozia et al. [38] on a gradual decrease of root and shoot fresh weight in Helianthus annus (L.) with increase in chromium level. The toxic effect of chromium on the root and shoot fresh weight in eight-day old seedling of Brassica oleracea (L.) var. acephala DC (kale) were reported by Ozdener et al. [39], treated with various concentrations of chromium in the growth medium.
4.5. Effect of Chromium Toxicity on Root and Shoot Dry Weight
The root and shoot dry weight of Hibiscus esculentus were decrease with an increase in chromium level (Table 3). Result shows that at 0.5, 2.5, 5, 10 and 5 mg·kg−1 chromium produced no significant (p > 0.05) effect on root dry weight 0.07 ± 0.02, 0.05 ± 0.00, 0.04 ± 0.00, 0.03 ± 0.00, 0.03 ± 0.01 respectively. The root dry weight of Hibiscus esculentus was significantly (p < 0.005) affected at 50 mg·kg−1 by 0.01 ± 0.00. Similarly, the average shoots dry weight of Hibiscus esculentus 0.11 ± 0.02, 0.09 ± 0.02, 0.09 ± 0.01, 0.05 ± 0.00, 0.05 ± 0.02 was not significantly (p > 0.05) affect at 0.5, 2.5, 5, 10 and 25 mg·kg−1 respectively. Although the dry weight of shoot was significantly (p < 0.05) affected at 50 mg·kg−1 by 0.02 ± 0.00. It was reported by Ganesh et al. [40] that there was a reduction in growth, dry weight in four genotypes of soybean at 5 - 200 mg·L−1 concentration of chromium, with respect to control application. In a study conducted on Vallisneria spiralis to evaluate the chromium accumulation and toxicity in relation to biomass production, Vajpayee et al. [41] was found that dry matter production affected by chromium concentration above 2.5 m·L−1 Ag in nutrient medium.
4.6. Effect of Chromium Toxicity on Chlorophyll Contents
The effects of chromium on photosynthetic pigments of Hibiscus esculentus leaves were determined on 30th day (Table 4). The photosynthetic pigment chlorophyll-achlorophyll-b and total chlorophyll of Hibiscus esculentus were decrease with increased chromium treatments (Figure 4). The chlorophyll-a in Hibiscus esculentus leaves were significantly (p < 0.05) decrease from 5.36 ± 0.05, 4.81 ± 0.04, 4.43 ± 0.05, 2.24 ± 0.06, 0.22 ± 0.00 and 0.19 ± 0.01 respectively. Similarly, the chlorophyll-b in Hibiscus esculentus leaves were decrease significantly (p < 0.05) from 8.73 ± 0.00, 3.55 ± 0.00, 3.47 ± 0.00, 1.68 ± 0.02, 1.41 ± 0.01, and 1.18 ± 0.00 respectively. The total chlorophyll content significantly (p < 0.05) affected at high chromium concentration from 14.09 ± 0.05, 8.37 ± 0.04, 7.90 ± 0.05, 3.92 ± 0.07, 1.64 ± 0.01, and 1.38 ± 0.01 respectively. Some other studies have the same conclusion that the chlorophyll contents were decrease if kept under Cr 6 + stress (10 - 40 mg L−1) [34].

Table 4. Effect of different chromium concentration on chlorophyll contents (a, b, total) in Hibiscus esculentus (L.).
The studies indicate that heavy metals and metalloids have effects on chlorophyll and amino acid content in plants. Heavy metals are known to interfere with chlorophyll synthesis either through direct inhibition of an enzymatic step or by inducing deficiency of an essential nutrient [42].
4.7. Effect of Chromium on Tolerance Index
The result shows that increasing level of chromium lower the percentage tolerance in Hibiscus esculentus (Table 5, Figure 5). The root length of Hibiscus esculentus at different chromium treatment 0.5, 2.5, 5, 10, 25 and 50 mg·kg−1 has 93.18%, 88.53%, 87.09%, 68.81%, 56.27% and 26.16% of tolerance respectively. The highest value (93.18%) of RLSTI was recorded at 0.5 mg·kg−1 and the lowest (26.16%) at 50 mg·kg−1 respectively. Similarly, tolerance level of shoot length in Hibiscus esculentus was decreased from 96.66%, 85%, 86.66%, 68.33%, 51% and 33.33% respectively due to increased chromium level. The maximum SLSTI value (96.66%) was recorded at 0.5 mg·kg−1 and the lowest (33.33%) at 50 mg·kg−1 respectively.
The RFSTI of Hibiscus esculentus was decrease as chromium level increased in the soil from 89.06%,

Figure 4. Effect of chromium on chlorophyll content in Hibiscus esculentus (L.).
89.06%, 78.12%, 76.56%, 60.93% and 51.56% respectively. The highest value (89.06%) for RFSTI was recorded at 0.5 mg·kg−1 and the lowest value (51.56%) at 50 mg·kg−1 respectively. Similarly, the SFSTI of Hibiscus esculentus.was also decreased from 96.01%, 90.43%, 83.66%, 47.41%, 37.45% and 16.73% respectively. The maximum value for SFSTI (96.01%) was recorded at 0.5 mg·kg−1 and the lowest value (16.73%) at 50 mg·kg−1 respectively.
The dry matter stress tolerance index (DMSTI) of Hibiscus esculentus was adversely affected with chromium application. The RDSTI of Hibiscus esculentus was decreased from 100%, 68.18%, 59.09%, 50%, 40.09% and 22.72% respectively. Maximum value for RDSTI (100%) was noted at 0.5 mg·kg−1 and the lowest value (22.72%) at 50 mg·kg−1 was recorded. Similarly, the SDSTI of Hibiscus esculentus was also decreased from 97.14%, 82.85%, 82.85%, 48.57%, 42.85% and 17.14% respectively. The maximum SDSTI value (97.14%) was recorded at 0.5 mg·kg−1 and the lowest value (17.14%) at 50 mg·kg−1 was recorded. A study on stress tolerance suggests that mechanism of tolerance helps plant to maintain growth even in the presence of potentially toxic metal concentrations [43,44] used the root and shoot
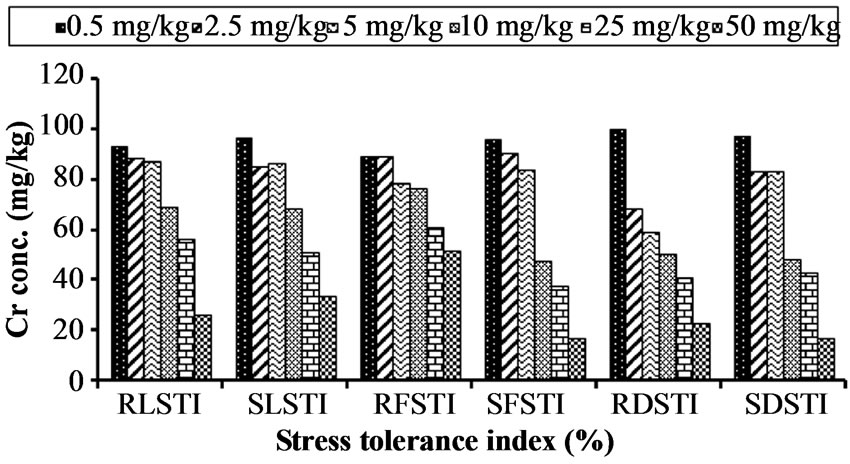
Figure 5. Effect of chromium on stress tolerance index (%) in Hibiscus esculentus (L.).

Table 5. Stress tolerance index (%) of Hibiscus esculentus (L.) grown in different treatments of chromium contaminated soil.
growth as an important parameter in classification of heavy metal tolerance.
Decrease in fresh weight because of low water uptake may be due to subsequent membrane damage since plant cell membrane generally considered as the primary sites of metal injury [45]. The negative effect of chromium on tolerance indices of fresh and dry weight was also determined within rice [46].
5. Conclusion
The toxic effect of heavy metal on plant growth depends on the amount of toxic metal taken up from the specified environment. The extensive use of chromium in a large number of products and industrial process has resulted in severe environmental contamination. Chromium toxicity has become significant due to its constant increase in the environment. Increasing concentration of chromium metal significantly inhibits seed germination, growth and biochemical attribute of Hibiscus esculentus. The overall inhibitory effect of chromium calculated as tolerance index which was more pronounced in Hibiscus esculentus seedlings. This information can be considered a contributing step in exploring and finding the tolerance limit of Hibiscus esculentus at different concentration of chromium. Results of the study are useful indicators of chromium tolerance to some extent for plantation of Hibiscus esculentus in chromium contaminated areas. However, in the toxic metal contaminated areas, further research is needed to determine the effect of different level of metals in the environment and various parts of the plant.
6. Acknowledgements
The authors would like to thank Institute of Plant Sciences, University of Sindh, Jamshoro, Pakistan for providing technical assistance of this work.
NOTES