1. INTRODUCTION
Barley crop in Mexico is basically oriented to malt elaboration for beer production [1-3]. Protein content in the grain is a factor that varies widely depending on both ecological and soil conditions of the place where it is grown, being such variable and generally considered as the most important index for malt quality prediction. There is a limitation on protein levels, since amounts lower than 10.5% in the most do not provide enough food for the yeast to develop properly, which derives in the occurrence of deficient alcoholic fermentations, as well as in contents above 13.5%, accompanied by turbidity in the most. Moreover, a poor grain in gums or carbohydrates glucocane type is demanded in order to have the most, easily separated from the grain [4].
The barley acreage is rather variable, having around 350,000 ha planted on a yearly basis. Its production is concentrated mainly in five states: Guanajuato (33.9%), Hidalgo (27.3%), Tlaxcala (14.6%), the State of México (7.9%) and Puebla (6.7%). The average annual production is 218,051 t, and for rainfed 372,569 t. A total of 94.1% barley is produced by Guanajuato in irrigation mode, whereas 97.3% is produced by Hidalgo in rainfed. Thus, barley production does not present a definite trend, as it is directly associated with yield and price volatility [5]. The sale of malt barley is usually carried out through contracts or agreements with “IMPULSORA AGRÍCOLA, S.A.” (IASA). IASA acts as mediator between producers and the brewery enterprise since 1958, providing credits collateral warranty and collateral with payment at the time of delivery. Seed supplying and crop management recommendations are provided by IASA; fertilizers, insecticides, herbicides and fungicides are acquired with local distributors [4].
One of the most important synergies for the country agricultural growth has been created by the link between barley demand and beer production, which consists on permanently fostering the development of an improved malting barley variety, aiming to achieve a high agricultural and industrial quality; such endeavor has been developed since 1961, when both the malting industry and the Instituto Nacional de Investigaciones Forestales Agrícolas y Pecuarias (INIFAP) celebrated the first cooperation agreement, aiming for the improvement of malting barley crop in the country. A major concern between IASA and INIFAP has recently arisen with regards to include the use of fertilizers in the barley production technological package, specifically barley seed mycorrhizal inoculated.
Since barley is highly susceptible to many fungal diseases, the best control is to create resistant varieties, even though there is a lack of varieties resistant to all of them; some of these, such as smut, coal dress, blotch and Fusarium, can be transmitted through the seed, turning fungicide treatment to the seed into a rather common practice [6]. A lack of knowledge concerning the symbiosis effectiveness among the pesticide-crop-biofertilizer specific interaction prevails because Mycorrhizal INIFAP® is a fungus and barley seed is treated with fungicides [7,8]. The one pesticide recommended by IASA is chlorothalonil. Consequently, the present study is aimed to evaluate the effect derived from the barley seed treatment with chlorothalonil fungicide on root colonization by Mycorrhizal INIFAP® at different storage times.
2. MATERIALS AND METHODS
The barley seed Esmeralda variety was treated with chlorothalonil, a contact fungicide both preventive and curative that controls a wide range of fungal diseases affecting vegetable, cereal, citrus and fruit crops. Such product is not toxic neither for mammals nor birds, but it is extremely toxic for fish.
The dose utilized was 250 ml for 100 kg seed; rhodamine dye was added looking forward to verify that the treatment was uniform. A total of four groups of 250 g fungicide treated seed and four more groups of 250 g untreated seed were separated after seven days; then, inoculation with Mycorrhizal INIFAP® containing a 40 spores∙g−1 soil concentration was performed; the dose used was 1 kg per 50 kg seed. Treatments are shown on Table 1.
Root colonization by Mycorrhizal INIFAP® percentages were evaluated at 0, 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 months of storage, with a technique modification of the mycorrhizal roots clearing and staining [9]. A germination test was performed on all of the treatments for each evaluation prior to sowing.
A completely randomized design with three replications was used; each experimental unit consisted of 12 oz styrofoam cups with methyl bromide previously steril-

Table 1. Treatments evaluated in the experiment.
ized substrate (mixed soil: sand 1:1); the planting density was 10 seeds per experiment unit. A destructive sampling of the experimental units was carried out 20 days after planting in order to have the 10 plants root colonization percentage determined [10].
Data were analyzed with a three factors variance analysis for a completely randomized design; the significant difference among means was determined at α < 0.05 through the Tukey test [11].
3. RESULTADS AND DISCUSSION
Significant differences were revealed in the colonization percentage for all of the analyzed variables, as shown on Table 2.
Significant differences were revealed concerning the average root colonization by Mycorrhizal INIFAP® in barley plants whose seed was treated with and without chlorothalonil (Table 3). The seed treatment with fungicide decreased root colonization by 2.42%; even though such decrease may seem low, it is the average time of all samplings and, as each sampling time is individually analyzed, greater differences shall be observed among treatments. Scientists have stated that fungicides affect the symbiosis of arbuscular mycorrhizal fungal (AMF) with the host plant in different manners including negatively, neutrally and positively [12]. Some fungicides have been shown to have deleterious effects on AMF when applied as a soil drench, it appears that the low rates needed for seed treatment dissipate sufficiently to allow for AMF to colonize seedlings during early growth [13].
The root colonization percentages by applying different Mycorrhizal INIFAP® doses are shown on Table 4; significant differences are observed among treatments. It shall be noted that the root colonization becomes increased by approximately 15% as the dose application is either double or triple, if compared with the recom-

Table 2. Mean squares of the analyzed variables.

Table 3. Average root colonization by Mycorrhizal INIFAP® in barley plants.

Table 4. Root colonization average using different mycorrhizal biofertilizer doses.
mended dose; nevertheless, there are no difference among treatments with two or three doses, which means that two doses are enough inoculum for maximum root infestation. Contrasting results were obtained for the pesticide effect on root colonization by mycorrhizal. As mentioned by Campagnac et al. [14], the colonization is affected by the fenpropimorph, negatively. Higher root colonization percentages were obtained by Hwang et al. [15], on the presence of metalaxyl fungicide in small doses. Powell et al. [16], on the other hand, have reported that root colonization by mycorrhizal in sorghum and soybean crops increases by applying glyphosate.
Root colonization results through storage time are presented on Table 5. Significant differences prevailed as inoculated seed (both treated and not treated with fungicide) storage time increases; effectiveness for root infection decreases progressively. The colonization decrease that occurred from the inoculation time, after 10 months storage time, was 26%.
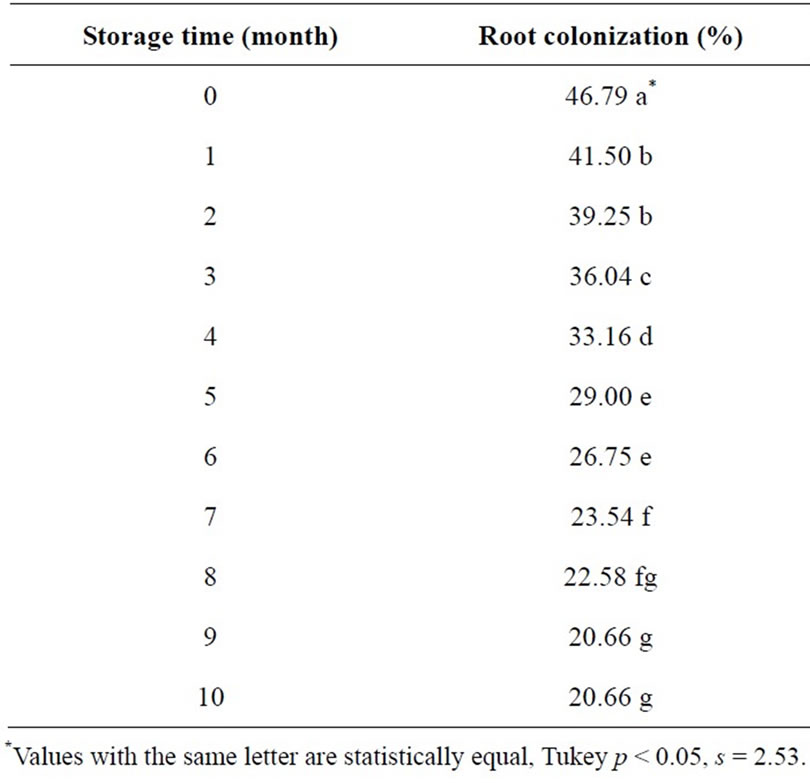
Table 5. Root colonization by Mycorrhizal INIFAP® in barley seed stored for 10 months.
This decrease is probably because the mycorrhizal inoculated in the seed is not under the same airproof storage conditions as in its original container, losing its viability, thus. Furthermore, it can also be observed that the seed germination rate declines overtime, decreasing in 20% within a 10-month period, being most affected when treated with chlorothalonil (Figure 1). As indicated by such data, it is not advisable to have the barley seed chlorothalonil treated, inoculated and stored for a long period of time. It is recommended to have the inoculation performed prior to planting or at least five months before as a maximum, looking forward to lose less than 50% effectiveness in colonization.
Regarding colonization percentage among biofertilizers doses with and without chlorothalonil treated seed, significant differences were revealed (Table 6). As observed, the colonization percentage was significantly decreased by the chlorothalonil application; however, there were no differences in root colonization percentage between the single dose and the two mycorrhizal doses, in neither fungicide treated nor untreated plant seeds. The concentration of chlorothalonil fungicide may also affect the symbiosis whith the host plant and decreased the colonization ratios [17].
The root colonization percentage was affected by the storage time for both fungicide treated and untreated seedlings (Table 7). As it can be observed, colonization effectiveness was reduced in 50% over a 10-month storage period (Figure 2).
Regardless the amount of mycorrhizal inoculant applied to the seed, as shown on Figure 3, the same colonization percentage is produced after 5 months storage in both treated and untreated fungicide seedlings. The My-

Figure 1. Germination loss through barley seed storage time treated with and without chlorothalonil.

Figure 2. Root colonization at different seed storage times.

Figure 3. Root colonization at different storage times, having 1, 2 and 3 Mycorrhizal INIFAP® doses applied.
corrhizal INIFAP® is composed by soil with spores, hyphae and mycorrhizal roots [18]. As revealed by the results, the seed germination percentage decreased and it was the same as that obtained after 10 months storage, when treated with chlorothalonil and inoculated with mycorrhizal; nonetheless, the root colonization percentage was the same at the beginning (time 0) of the test. As indicated by these results, the mycorrhizal inoculated to the seed loses viability as storage goes by, and some biofertilizer components (spores, hyphae or mycorrhizal

Table 6. Interaction among biofertilizers doses and root colonization.

Table 7. Effect of the storage time on root colonization by Mycorrhizal INIFAP®.
rootlets) certainly fall short in effectiveness due to aeration and change exposure in humidity or compounds creation produced by the seed in storage; however, a lack of reports in such regard currently prevail (Figure 4).
4. CONCLUSIONS
The root colonization by Mycorrhizal INIFAP® percentage was significantly decreased by 2.42% by the seed treatment with chlorothalonil; still, since this assessment was carried out after 20 days, such decrease shall be recovered as the crop develops itself. The most affected treatment, with regards to root colonization by Mycorrhi-
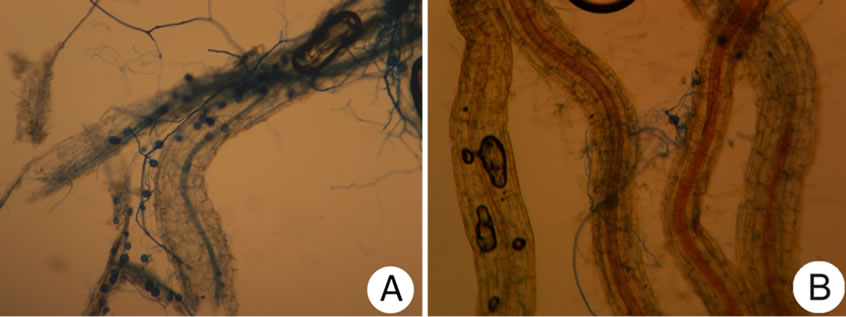
Figure 4. Root colonization by Mycorrhizal INIFAP® in barley roots whose seed was treated with chlorothalonil. (A) Without storage; (B) With 10 months of storage.
zal INIFAP® with chlorothalonil treated was the treatment conducted with a single dose. No significant differences were revealed by neither the double nor the triple doses treatments.
The germination percentage was significantly decreased by the seed storage time, progressively and directly related with storage time, not withstanding if treated with fungicide and mycorrhizal. Throughout the inoculated seed storage time, the root colonization by Mycorrhizal INIFAP® decreases by 50% within a 10 month period, even if it is fungicide treated or not. Thus, it is suggested to double the inoculation dose with Mycorrhizal INIFAP® as the barley seed is treated with chlorothalonil.
5. ACKNOWLEDGEMENTS
To the SAGARPA Management of Bio-economics for the support granted to carry out the study hereby through the Grant No. S2341- HA4310111.