Effect of Proline Pretreatment on Grapevine Shoot-Tip Response to a Droplet-Vitrification Protocol ()
1. Introduction
Proline has been shown to accumulate in plant cells and organs in response to biotic (plant-pathogen interaction) and abiotic (water limitation, redox potential modification, freezing, etc.) stresses, even though this amino acid seems to accumulate in highest concentrations in the event of a drought stress [1]. These stress-mitigating properties have prompted the inclusion of exogenous proline in some plant cryopreservation protocols in order to improve explant survival and regrowth [2]. Although its modes of action are not fully known, proline could likely act protectively during the dehydration steps included in all cryopreservation protocols. Indeed, dehydration is known to evoke the formation of reactive oxygen species [3] and exogenous proline has recently been shown to alleviate H2O2-mediated oxidative stress in grapevine leaves [4].
Thus, while satisfactory efficiency of cryopreservation is documented for in vitro grapevines [5], further improvement could be expected if stresses linked to cryopreservation procedures (such as oxidative stress) could be reduced. To our knowledge the possible beneficial effect of proline in grapevine cryopreservation has not been tested yet and we therefore considered it worthwhile to investigate its influence in a vitrification-based protocol.
2. Materials and Methods
Single-node explants isolated from in vitro-grown grapevine plantlets (Vitis vinifera L. cv Portan) were cultured for 2 weeks on shooting media (half-strength MS-based medium plus 1 µM BAP) containing no proline (control) or 50, 500, or 2000 µM filter-sterilized L-proline.
After 2 weeks of culture of single-node explants (at least 37 explants per experimental condition) on the different shooting media tested, the number of shoot-developing nodes was counted and individual shoot length and number of newly-developed nodes were recorded. Shoot tips excised from micro-shoots obtained on the different shooting media were subjected to a PVS2-based droplet-vitrification protocol [6] and plunged into liquid nitrogen (LN) for at least 1 h. Control explants (treated with loading, PVS2, and unloading solutions, but not exposed to LN) and LN-treated, rewarmed explants were grown on the same recovery medium containing 1 µM BAP. Thirteen to eighteen explants were used per condition.
3. Results and Discussion
3.1. Effect of Proline on Shoot Development and Growth Parameters
Shoots developed on the different shooting media did not demonstrate notable differences in aspect (Figure 1) although leaves produced on media with the higher two
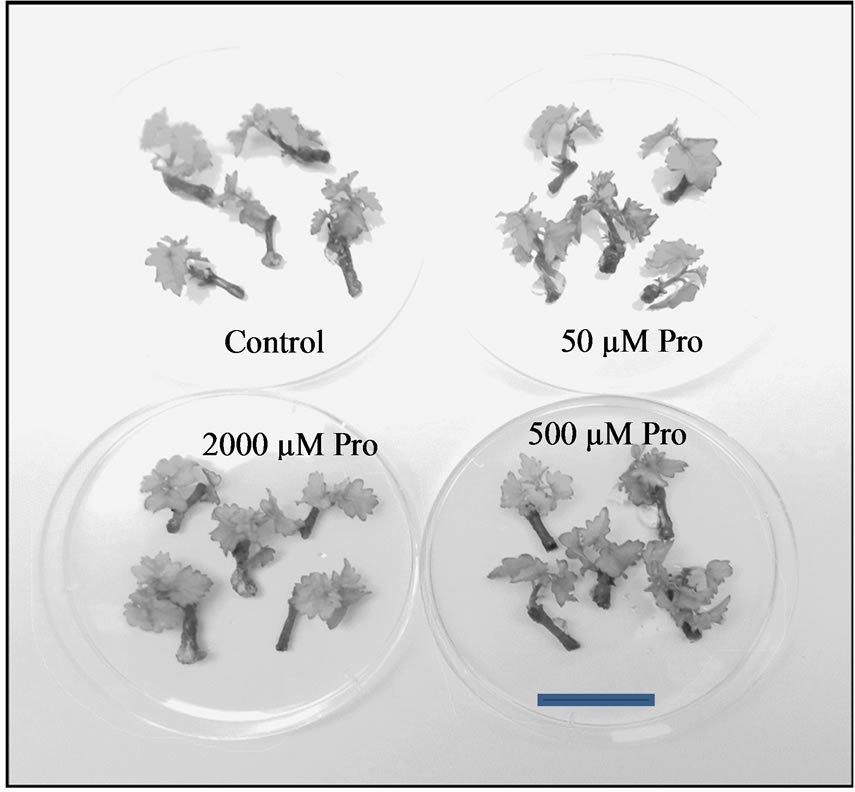
Figure 1. Representative samples of shoots developed after 2 weeks on different shooting media. Bar = 2 cm.
proline concentrations displayed a slightly lighter green; similarly, a comparable number of shoots and nodes were produced over the range of media, although a slightly lower number of nodes were formed on the medium containing the highest proline concentration (Figure 2).
Shoots arising from the control medium and media with the lower two proline concentrations did not notably differ in their development with similar shoot length measured, whereas the highest proline concentration inhibited shoot development (Figure 3) with a significant global difference between treatments. As no other pairwise comparisons were significant, this could be explained by a slightly enhancing effect of proline used at 500 µM and a slightly depressive effect of the 2000 µM treatment, both not large enough to differ from the control but statistically different when compared. Thus, in our conditions, proline did not markedly alter shoot development although some toxicity could be suspected at high concentrations. Indeed, excess proline has been shown to negatively affect chlorophyll-binding proteins [7], which could account for the loss of coloration observed at the highest two proline concentrations. In addition, both the number of nodes formed and micro-shoot length mean value were lower (but not significantly)

Figure 2. Number of shoots developed and total number of nodes formed 2 weeks after inoculation on the different shooting media.

Figure 3. Mean shoot length (mm) obtained on the different shooting media with a = 5% confidence intervals. Mean values differing by one letter are significantly different (Student’s t-test, a = 5%).

Table 1. Percentage of survival and regrowth (observed after 2 and 8 weeks, respectively) of control (−LN) and cryopreserved (+LN) shoot tips excised from different shooting media and subjected to a PVS2-based droplet-vitrification protocol.
when explants were grown at the highest proline concentration.
3.2. Effect of Proline on Post-Cryopreservation Survival and Regrowth
After explant pretreatment with loading/PVS2 solutions with or without LN exposure, shoot tip survival and regrowth were recorded after 2 and 8 weeks (Table 1). Carry-over effects for the 2000 μM proline medium were observed since lower survival and regrowth were obtained both for LN-treated or non-treated explants excised from micro-shoots obtained on this medium. No significant differences in survival and regrowth were observed for explants subjected to pretreatment phases without LN exposure. A slightly enhancing effect (although non-significant) on post-cryopreservation survival was observed for explants derived from shoots developed on 50 or 500 μM proline, but no significant improvement of regrowth was observed for these two conditions.
Increasing explant number in repeat experiments would clearly improve statistical significance. However, the 2-week period before explant excision could have allowed at least partial catabolism of exogenous proline back to glutamate [8]. Exposure to exogenous proline at higher (45 mM) concentration has also been shown to modify the endogenous amino acid pool [9]. We therefore cannot rule out the fact that interpretation of the results observed in our conditions could have been made difficult as a result of complex interactions. Shorter exposure to proline can then be envisaged in order to reduce proline catabolism and better evidence its proper effect. The actual rate of proline uptake by in vitro grapevine explants is not known, but endogenous proline content of Arabidopsis seedlings was increased almost 100-fold within 24 h when they were treated with 45 mM proline [9] and a 6-h treatment with 20 mM proline was sufficient to effectively reduce oxidative stress in grapevine leaves excised from in vitro plants [4]. It is therefore likely that shorter proline treatments applied just before LNexposure (i.e. in the 24-h period after explant excision and before explant pretreatment with vitrification solutions) could evoke a clearer response as to the possible protective effect of proline during grapevine in vitro shoot tip cryopreservation.
4. Conclusion
Over the range of concentrations tested, proline did not strongly alter shoot development. Detrimental carry-over effects of proline at 2000 µM could be suspected as shoot tips regrowth after vitrification treatments and LN exposure was negatively affected. After LN exposure, although a slight increase in survival could be observed at the lowest concentrations tested, no significant effect of proline pretreatment on post-cryopreservation regrowth could be evidenced in our conditions. However, repeating the experiment with shorter durations of exposure to proline would likely allow clearer interpretation by limiting the effects of proline metabolism and likely interactions resulting therefrom.
5. Acknowledgements
This work has been supported by grants from the French Ministry of Foreign Affairs (Z. Marković) and from ARCAD, a flagship programme of Agropolis Fondation (Montpellier, France) (I. Engelmann-Sylvestre).