Phytochemical Constituents and Determination of Resveratrol from the Roots of Arachis hypogea L. ()
1. Introduction
Peanut (Arachis hypogea L.) belongs to the Fabaceae family. Being native to Central America, it is now also widely planted in Vietnam. It has been considered not only as a highly nutritious food source for human beings but also a natural starting material for the production of soap and machine oil. In addition, Arachis hypogea L. has been used in Vietnamese folk remedies for the treatments of various diseases such as loose cough, arthritis, constipation, recuperation after illness, etc. [1]. Most of the reported usages of peanut mentioned above were originated from the aerial part of the plant and seed, while much less attention to the root. Up to now, peanut roots have just been used as poultry feed and fertilizer without sufficient scientific knowledge [1,2].
In this paper, our work has studied on the phytochemical constituents of peanut roots via screening antioxidant activity of some different polarity fractions. In particular, resveratrol, a novel anti-cancer, was determined in peanut roots. The amount of resveratrol in their stems, leaves, roots was also studied to afford the information.
2. Experimental
2.1. General
Optical rotations were measured with an automatic digital Krüss P8000 polarimeter (Na lamp, 589 nm). NMR spectra were recorded on a Bruker Avance III spectrometer (500 MHz for 1H and 125 MHz for 13C) using residual solvent signal as internal reference: methanol-d4 δH 3.31, δC 49.15. Mass spectra were taken on a high resolution ESI Bruker Daltonics micrOTOF-Q II 10,187 mass spectrometer. Chromatography column was carried out on Kieselgel 60 (63 - 200 mesh) Merck and Li Chroprep Rp-18 (25 - 40 mm) Merck. Thin-layer chromatography was performed on Merck 25DC-Alufolien 20 × 20 cm Kieselgel 60 F254 and Merck RP-18 F254 plates. HPLC analyzes were carried out on a HPLC, Agilent 1100 Seri US, column C18-ACE (150 × 4.6 mm, 3 mm, l 303 nm).
2.2. Plant Material
Arachis hypogea L. was collected at Vang Quoi Tay commune, Binh Dai district, Ben Tre Province, Vietnam in September 2010 and was identified by lecturer Phan Gia Tan (Nong Lam University Hochiminh City). A voucher sample, coded as US-C017 was deposited on the Herbarium of the Organic Chemistry Department, Ho Chi Minh City University of Science.
2.3. Extraction and Isolation
The roots of the plants were washed, dried at 60˚C, ground into powder (1.6 kg) and extracted with methanol by maceration at room temperature. After evaporating, the methanolic filtrated solution gave crude extract (120 g). This crude extract was subjected to the silica gel solid phase extraction, and then successively eluted with hexane, chloroform, ethyl acetate and methanol to afford four different extracts: hexane, chloroform, ethyl acetate and methanol. These extracts were used to study antioxidant activity. Some extracts were chromatographed repeatedly to afford eight pure compounds: (1), (2), (3) from the chloroform extract; (4), (5) from the ethyl acetate extract and (6), (7) from the methanol extract (Figure 1).
Ursolic Acid (1)
White powder. Mp: 289˚C - 291˚C (chloroform). Rf: 0.50 (CHCl3-MeOH, 95:5).
1H-NMR (500 MHz, Acetone-d6): 5.24 (1H, m, H12), 3.20 (1H, dd, J = 10.0, 6.0 Hz, H3), 2.20 (1H, d, J = 11.5 Hz, H18), 1.09 (3H, s, H27), 0.98 (3H, s, H23), 0.95 (3H, d, J = 6.5 Hz, H30), 0.92 (3H, s, H25), 0.86 (3H, d, J = 6.5 Hz, H29), 0.81 (3H, s, H26) and 0.78 (3H, s, H24).
13C-NMR (125 MHz, Acetone-d6): 39.1 (C1), 27.9 (C2), 78.7 (C3), 37.4 (C4), 55.9 (C5),18.9 (C6), 33.6 (C7), 40.0 (C8), 48.1 (C9), 38.5 (C10), 23.7 (C11), 126.3 (C12), 139.0 (C13), 42.5 (C14), 28.0 (C15), 25.0 (C16), 48.3 (C17), 53.3 (C18), 39.0 (C19), 38.9 (C20), 31.2 (C21),37.4 (C22), 28.7 (C23), 15.9 (C24), 16.1 (C25), 17.3 (C26), 23.9 (C27), 178.9 (C28), 17.6 (C29) and 21.4
(C30).
Kaempferol (2)
Yellow powder. Mp: 275˚C - 277˚C (methanol). Rf: 0.40 (CHCl3 - MeOH, 9:1).
1H-NMR (500 MHz, Acetone-d6): 8.10 (2H, dd, J = 7.0, 2.0 Hz, H2’ and H6’), 6.93 (2H, dd, J = 7.0, 2.0 Hz, H3’ and H5’), 6.41 (1H, d, J = 2.0 Hz, H8) and 6.20 (1H, d, J = 2.0 Hz, H6).
13C-NMR (125 MHz, Acetone-d6): 147.1 (C2), 136.6 (C3), 176.6 (C4), 162.4 (C5), 99.3 (C6),165.2 (C7), 94.6 (C8), 158.0 (C9), 104.2 (C10), 123.8 (C1’), 130.5 (C2’), 116.4 (C3’), 160.3 (C4’), 116.4 (C5’) and 130.5 (C6’).
β-Sitosterol 3-O-β-D-glucopyranoside (3)
White powder. Mp: 284˚C - 286˚C (methanol). Rf: 0.30 (CHCl3-MeOH, 9:1).
1H-NMR (500 MHz, DMSO-d6): 5.32 (1H, brd, J = 5.0 Hz, H6), 4.22 (1H, d, J = 8.0 Hz, H1’) and 3.47 (1H, m, H3).
13C-NMR (125 MHz, DMSO-d6): 36.9 (C1), 30.0 (C2), 77.1 (C3), 38.2 (C4), 150.0 (C5), 121.2 (C6),31.4 (C7), 31.5 (C8), 49.7 (C9), 36.2 (C10), 20.6 (C11), 38.4 (C12), 41.9 (C13), 56.2 (C14), 23.9 (C15), 27.8 (C16), 55.5 (C17), 11.7 (C18), 19.1 (C19), 35.5 (C20), 18.6 (C21), 33.4 (C22), 25.6 (C23), 45.2 (C24), 28.8 (C25), 19.7 (C26), 18.9 (C27), 22.7 (C28), 11.8 (C29), 100.8 (C1’), 73.5 (C2’), 76.8 (C3’), 70.2 (C4’), 76.7 (C5’) and 61.2 (C6’).
Resveratrol (4)
White powder. Mp: 289˚C - 291˚C (methanol). Rf: 0.35 (CHCl3-MeOH, 7:3).
1H-NMR (500 MHz, CD3OD): 7.34 (2H, dd, J = 8.5, 2.0 Hz, H2’ and H6’), 6.95 (1H, d, J = 16.0 Hz, H8), 6.79 ( 1H, d, J = 16.5 Hz, H7), 6.76 (2H, dd, J = 9.0, 2.0 Hz,
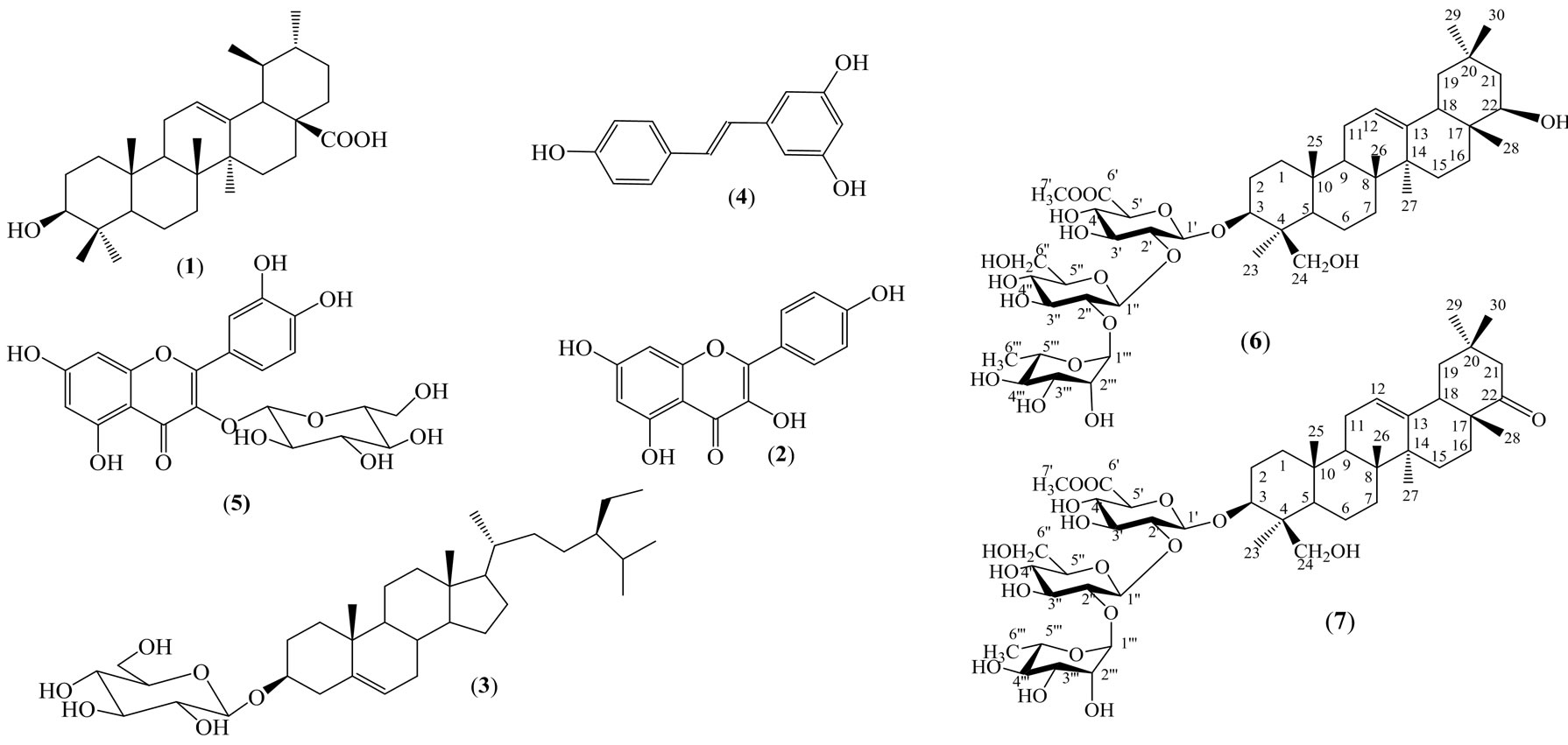
Figure 1. Compounds isolated from the root of Arachis hypogea L.
H3’ and H5’), 6.46 (2H, d, J = 2.0 Hz, H2 and H6) and 6.18 (1H, t, J = 2.0 Hz, H4).
13C-NMR (125 MHz, CD3OD): 141.4 (C1), 105.9 (C2, C6), 159.6 (C3, C5), 102.7 (C4), 129.5 (C7), 127.1 (C8), 130.4 (C1’), 128.8 (C2’, C6’), 116.5 (C3’, C5’) and 158.4 (C4’).
Quercetin 3-O-β-D-glucopyranoside (5)
Yellow powder. Mp: 195˚C - 197˚C (methanol). Rf: 0.30 (CHCl3-MeOH, 9:1).
1H-NMR (500 MHz, CD3OD): 7.71 (1H, d, J = 2.0 Hz, H2’), 7.58 (1H, dd, J = 8.5, 2.0 Hz, H6’), 6.87 (1H, d, J = 8.5 Hz, H5’), 6.39 (1H, d, J = 2.0 Hz, H8), 6.20 (1H, d, J = 2.0 Hz, H6), 5.24 (1H, d, J = 7.5 Hz, H1”), 3.76 (1H, dd, J = 11.5, 2.5 Hz, H6”a), 3.62 (1H, dd, J = 12.0, 5.5 Hz, H6”b), 3.53 (1H, d, J = 9.0 Hz, H4”), 3.48 (1H, d, J = 8.5 Hz, H3”), 3.39 (1H, t, J = 9.0 Hz, H2”), 3.28 (1H, ddd, J = 9.5, 5.0, 2.5 Hz, H5”).
13C-NMR (125 MHz, CD3OD): 158.5 (C2), 135.7 (C3), 179.5 (C4), 163.1 (C5), 99.9 (C6), 166.0 (C7), 94.7 (C8), 159.1 (C9), 105.7 (C10), 123.2 (C1’), 117.6 (C2’), 145.9 (C3’), 149.9 (C4’), 116.0 (C5’), 123.2 (C6’), 104.4 (C1”), 78.2 (C2”), 75.8 (C3”), 71.3 (C4”), 78.4 (C5”) and 62.6 (C6”).
3-O-{α-L-Rhamnopyranosyl-(1(2)-β-D-glucopyranosyl-(1(2)-6’-O-methyl-β-D-glucuronopyranosyl}so-yasapogenol B (6)
White amorphous powder. Rf: 0.30 (CHCl3 - MeOH, 7:3).
HR-ESI-MS (+) m/z 979.5233 [M + Na]+, calcd. for [C49H80O18+Na]+ 979.5242.
1H-NMR (500 MHz, CD3OD) and 13C-NMR (125 MHz, CD3OD): Table 1.
3-O-{α-L-Rhamnopyranosyl-(1(2)-β-D-glucopyranosyl-(1(2)-6’-O-methyl-β-D-glucuronopyranosyl}so-yasapogenol E (7)
White amorphous powder. Rf: 0.30 (CHCl3 - MeOH, 7:3).
HR-ESI-MS(+) m/z [M+Na]+ 977.5061, calcd. for [C49H78O18 + Na]+ 977.5086.
1H-NMR (500 MHz, CD3OD) and 13C-NMR (125 MHz, CD3OD): Table 1.
2.4. DPPH Free-Radical Scavenging Activity Assay
The stable free radical 1,1-diphenyl-2-picrylhydrazyl (DPPH) was used to determine the potential scavenging activity of different extracts, according to Molyneux P. method [3]. Extracts were diluted in DMSO and absolute MeOH to give a stock solution with final concentration of 1 mg/mL. These stock solutions were diluted into different concentrations (0.1 - 1.0 mg/mL) and were tested in triplicate in the same manner. 0.5 mL of this tested solution was incubated in 0.5 mL DPPH (0.6 mM) in 0.3 mL absolute MeOH at room temperature for 30 minutes in the dark. DMSO in MeOH was used as the negative control and vitamin E as the positive control. Absorbance was measured at 516 nm, and the percent scavenging activity was determined by comparison with the negative control as the following formula:
% scavenging activity = [(AN – AT)/AN] × 100%.
(AN: OD516 of negative control.
AT: OD516 of tested sample).
The fifty percent effective concentration (EC50) values were obtained through extrapolation from linear regression analysis.
2.5. Determination of Resveratrol in Stems, Leaves and Roots of Arachis hypogaea by HPLC
The roots, stems and leaves of the plants at 30 days, 60 days, 90 days old were washed, dried at 60˚C, ground into powder. Each specimen (50 g) was refluxed in ethanol-water (8:2) in 3 hours (500 mL × 3). The filtrated solutions were collected and were evaporated under the reduced pressure to obtain crude extract. The content of resveratrol in each extract (1 g) was analysed by HPLC, equipped with a UV detector set at 303 nm. The mobile phase (methanol-acetate buffer) was delivered at a flowrate of 0.5 mL/min.
Resveratrol was isolated from the roots of Arachis hypogea L. and its chemical structure was identified by NMR spectra as well as by comparison with the literature. It was used as a standard with the purity of about 99%.
The standard resveratrol and the extracts were separately and exactly weighted. Each sample was dissolved in 5 ml methanol. Then 0.5 ml of each solution was pooled out and diluted by methanol to 5 ml. These solutions were analyzed by HPLC. The content of resveratrol in the roots. stems and leaves was determined based on peak area of corresponding pure standards, with the fomula (Figure 2).
The content of resveratrol in sample = (SRes in Sample × mRes standard)/(SRes standard × mSample) × 106 (µg/g)
SRes in Sample: Peak area of resveratrol in sample.
SRes standard: Peak area of standard resveratrol.
mRes standar: Mass of standard resveratrol.
mSample: Mass of sample.
3. Results
Five known compounds as ursolic acid (1) [4], kaempferol (2) [5], β-sitosterol 3-O-β-D-glucopyranoside (3) [6], resveratrol (4) [7], quercetin 3-O-β-D-glucopyranoside (5) [8], and two triterpenoid saponins as 3-O-{α-Lrhamnopyranosyl-(1®2)-β-D-glucopyranosyl-(1®2)-6’- O-methyl-β-D-glucurono-pyranosyl}soyasapogenol B (6), 3-O-{α-L-rhamno-pyranosyl-(1®2)-β-D-glucopyranosyl-(1®2)-6’-O-methyl-β-D-glucuronopyranosyl} soya-
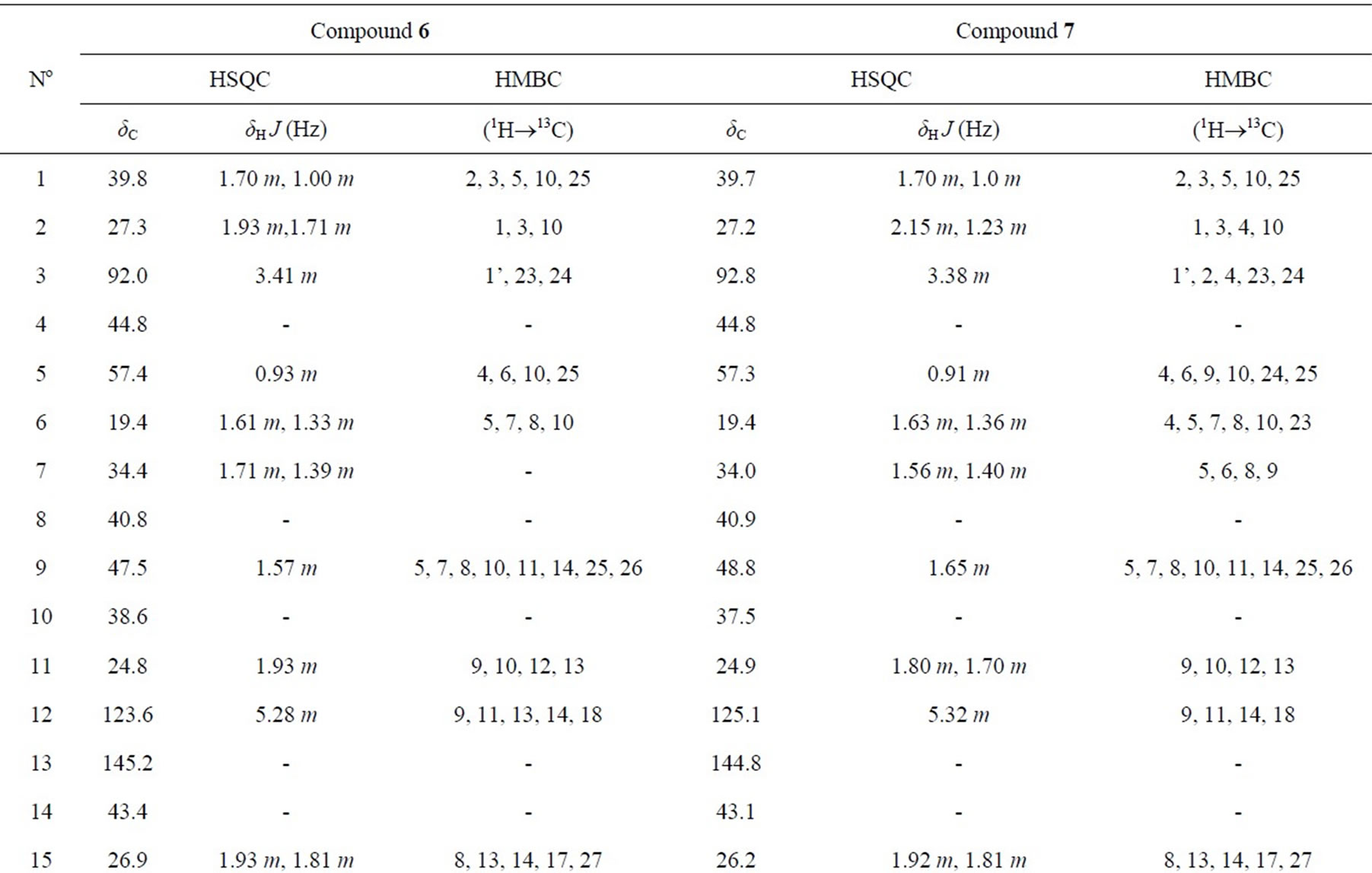
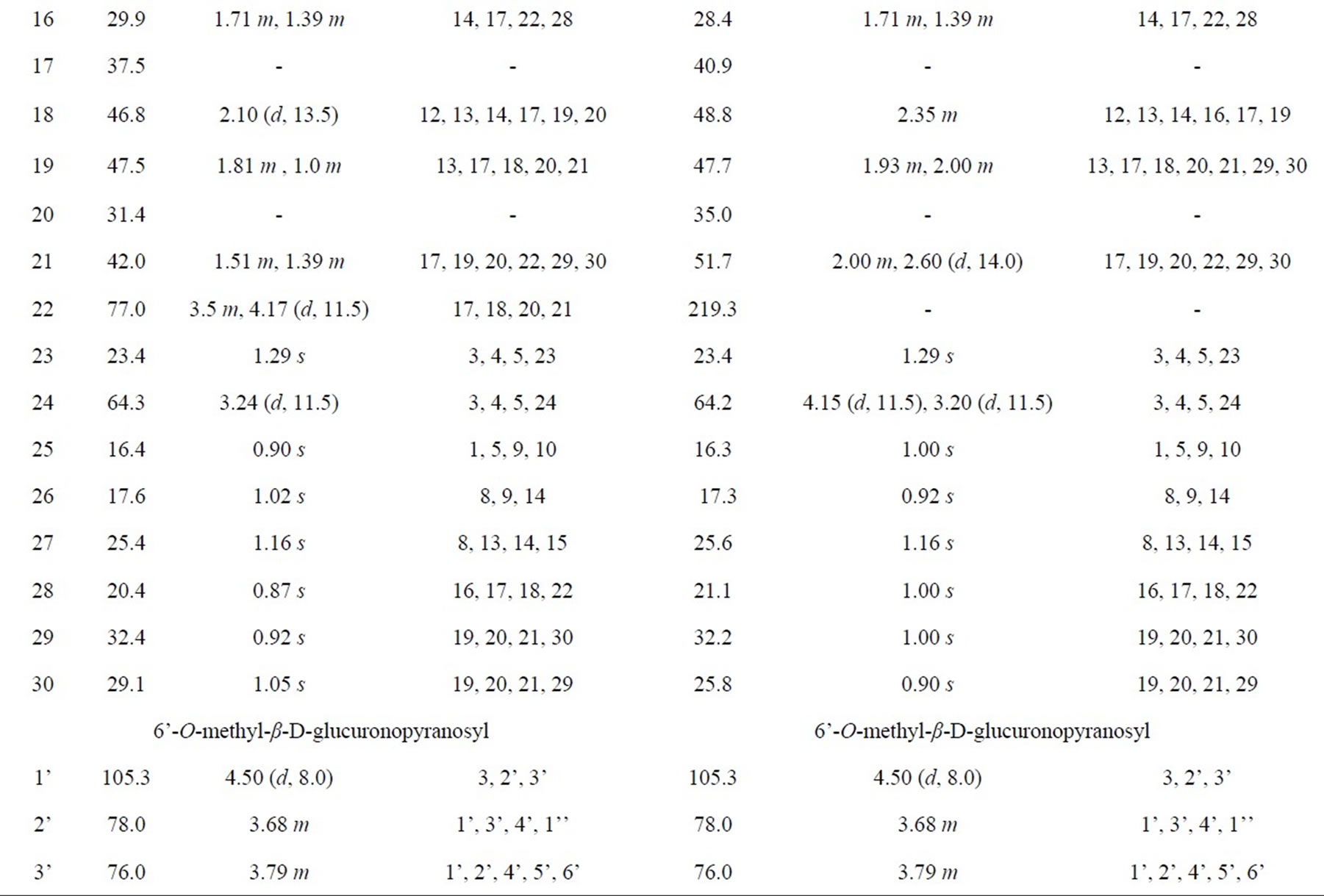
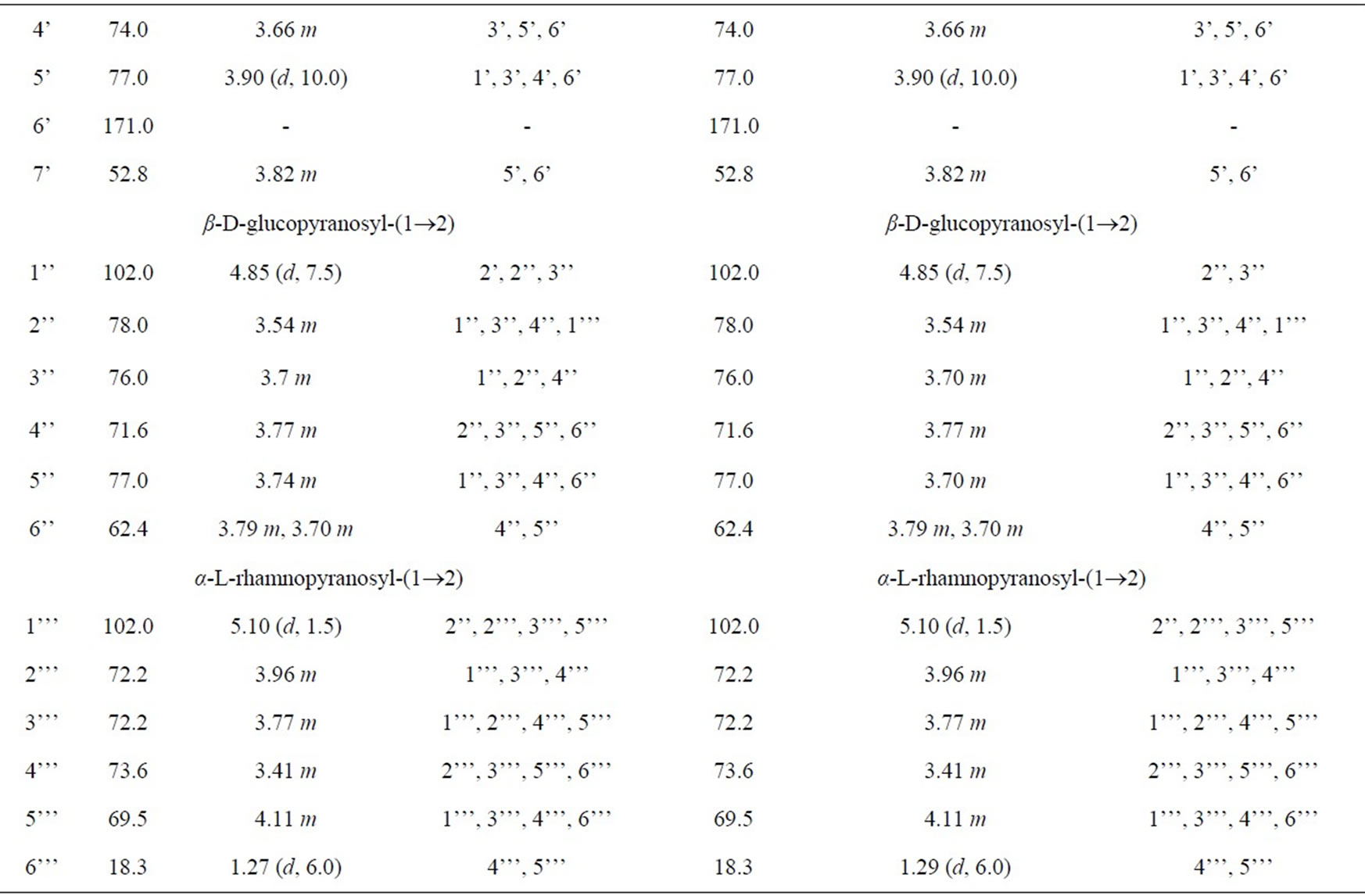
Table 1. NMR spectra of 6 and 7 (500 MHz for 1H and 125 MHz for 13C in CD3OD).
sapogenol E (7) [9] were identified by detailed NMR spectroscopic analysis and comparison of these data with the ones in the literature. This is the first time that all these compounds have been isolated from Arachis hypogaea L.
Antioxidant activity of four extracted fractions, obtained by DPPH assay [10], was shown in Table 2.
The level of resveratrol in different parts of Arachis hypogea L was determined. The result was displayed in Table 3.
4. Discussion
4.1. Structural Elucidation of Some Pure Compounds from Peanut Roots
Compound 6 was isolated as a white amorphous powder. Its molecular formula of C49H80O18 was determined from data of the positive-ion high-resolution-electrospray ionization mass spectrum (HR-ESI-MS) (m/z 979.5233 [M + Na]+, calcd. for [C49H80O18 + Na]+ 979.5242). The NMR spectra (Table 1) suggested that 6 was a triterpenoid saponin. The two olefinic carbon signals at δC 123.6 (C12) and 145.2 (C13), together with three carbinol carbon signals at δC 92.0 (C3), 77.0 (C22) and 64.3 (C23) confirmed the presence of soyasapogenol B aglycone which was glycosylated at C3.
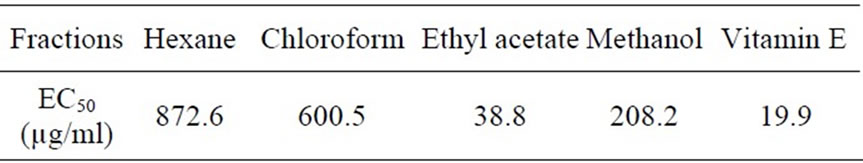
Table 2. Radical scavenging activity against DPPH of fractions extracted from the peanut roots.
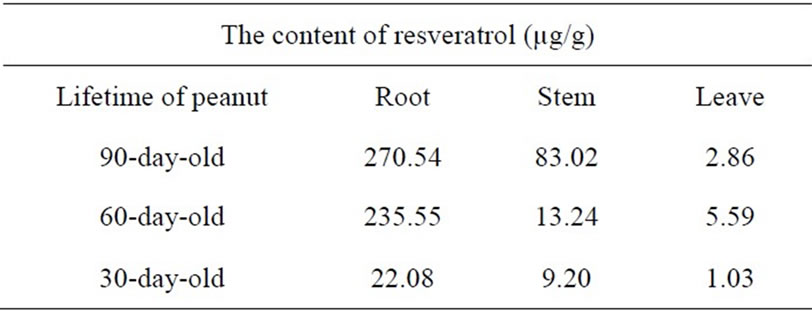
Table 3. The content of resveratrol in roots, stems and leaves of Arachis hypogea L.
The 1H and 13C-NMR spectra indicated the presence of α-L-rhamnopyranosyl, β-D-glucopyranosyl and 6’-Omethyl-β-D-glucuronopyranosyl moieties with their anomeric protons and carbons resonances at δH 4.50 (d, J = 8.0 Hz, GlcA-H1’) and δC 105.3 (GlcA-C1’), δH 4.85 (d, J = 7.5 Hz, Glc-H1”) and δC 102.0 (Glc-C1”), δH 5.10 (d, J = 1.5 Hz, Rha-H1’”) and δC 102.0 (Rha-C1’”), respectively. The HMBC correlations between GlcA-H7’ (δH 3.82) and GlcA-C6’ (δC 171.0) showed that the
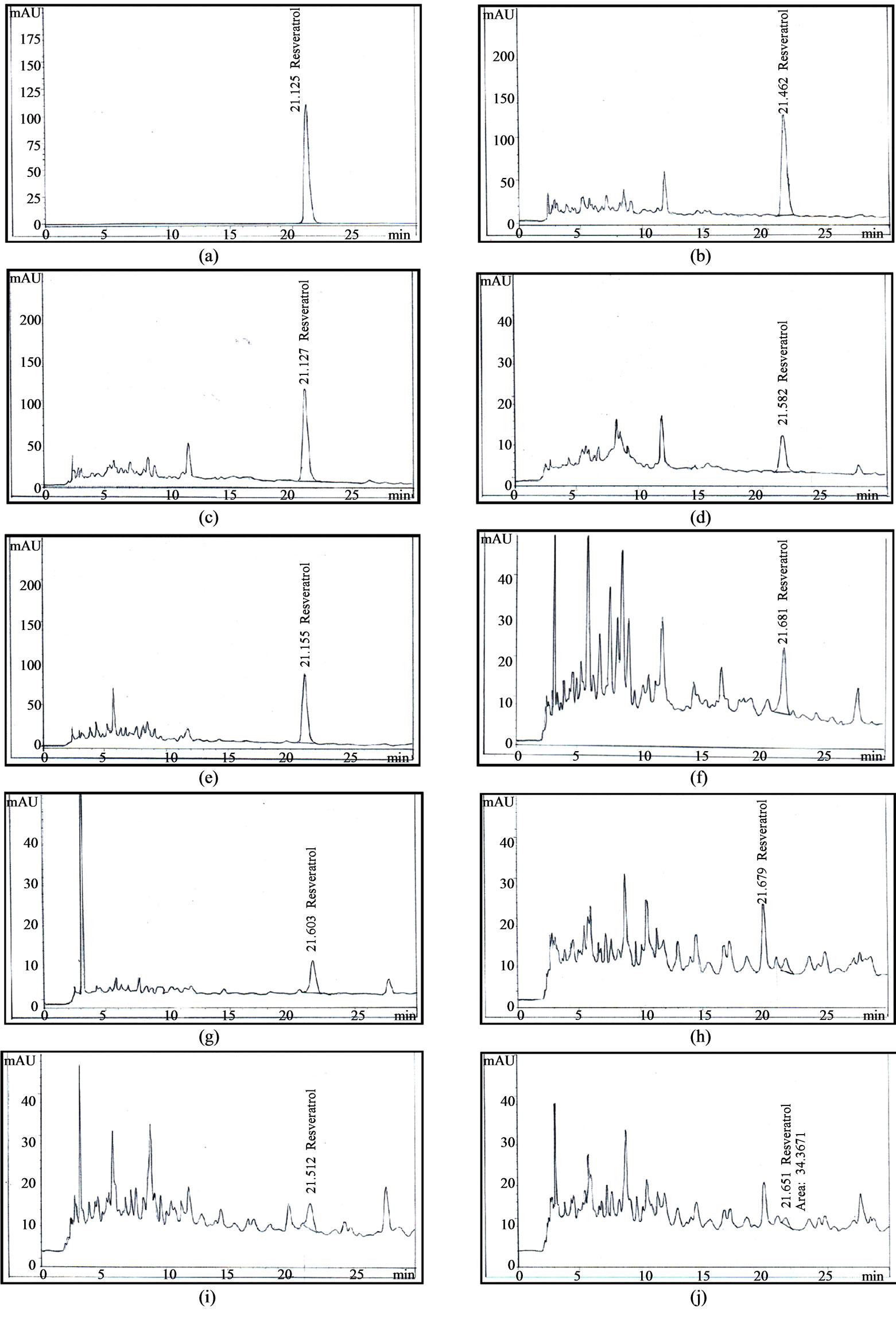
Figure 2. HPLC chromatogram of resveratrol from the roots, stems and leaves of Arachis hypogaea L. (a) Standard resveratrol; (b) 90-day-old roots (c) 60-day-old roots; (d) 30-day-old roots; (e) 90-day-old stems; (f) 60-day-old stems; (g) 30-day-old stems; (h) 90-day-old leaves; (i) 60-day-old leaves; (j) 30-day-old leaves.
β-D-glucurono-pyranosyl moiety was methyl esterified. Furthemore, the HMBC correlations between Rha-H1’” (δH 5.10) and Glc-C2” (δC 78.0), Glc-H1” (δH 4.85) and GlcA-C2’ (δC 78.0), GlcA-H1’ (δH 4.50) and C3 (δC 92.0) established the attachment of these sugars and the sugar moiety with the aglycone moiety. Therefore, 6 was determined as 3-O-{α-L-rhamnopyranosyl-(1®2)-β-Dglu-copyranosyl-(1®2)-6’-O-methyl-β-D-glucuronopyranosyl}soyasapogenol B, named as azukisaponin V methyl ester, isolated from Trifolium alexandrinum [9].
Compound 7 was isolated as a white amorphous powder. Its molecular formula C49H78O18 was determined from the HR-ESI-MS (m/z [M + Na]+ 977.5061, calcd. for [C49H78O18 + Na]+ 977.5086). The NMR data of sugar moieties of 6 and 7 were similar. Comparison of the NMR spectroscopic data of aglycone moieties of 6 and 7 showed superimposable resonances, except for the appearance of ketone group at δC22 219.3 ppm in 7 super seded the hydroxyl group at δC22 77.0 ppm in 6 (Table 1). 7 was concluded as 3-O-{α-L-rhamnopyrano-syl-(1®2)- β-D-glucopyranosyl-(1®2)-6’-O-methyl-β-D-glucurono pyranosyl}soyasapogenol E named as dehydroazukisaponin V methyl ester. This compound was also isolated from Trifolium alexandrinum [9].
4.2. Antioxidant Activity of Fractions Extracted from Peanut Roots
The result in Table 2 showed that the ethyl acetate fraction, containing resveratrol (4), quercetin 3-O-β-D-glucopyranoside (5) was the most active in the radical scavenging activity against DPPH. Further studies on the purification and identification of components responsible for the antioxidant activity of ethyl acetate and methanol fractions are now in progress.
4.3. Content of Resveratrol in Peanut Roots
Recent researches showed that resveratrol was found in peanut and this compound could inhibit colon, prostate and breast cancer; to prevent heart disease and diabetes type 2, arthritis, and to reduce the risk of Azheimer’s disease [10-13]. Due to the potential values of resveratrol, we focused on determining the level of resveratrol in different parts of Arachishypogea L. to find out some useful informations for the recycling of peanut roots that have just been used a by-product, i.e. fertilizer.
The content of resveratrol in the roots was always higher than that in the stems and leaves, especially highest in the roots of 90 days old (Table 3). The farmer usually collected the peanut after 3 months and throw away the roots. This result suggests the possibility of recycling the wasted peanut roots from which resveratrol could be extracted.
The summary of data collected from other publications
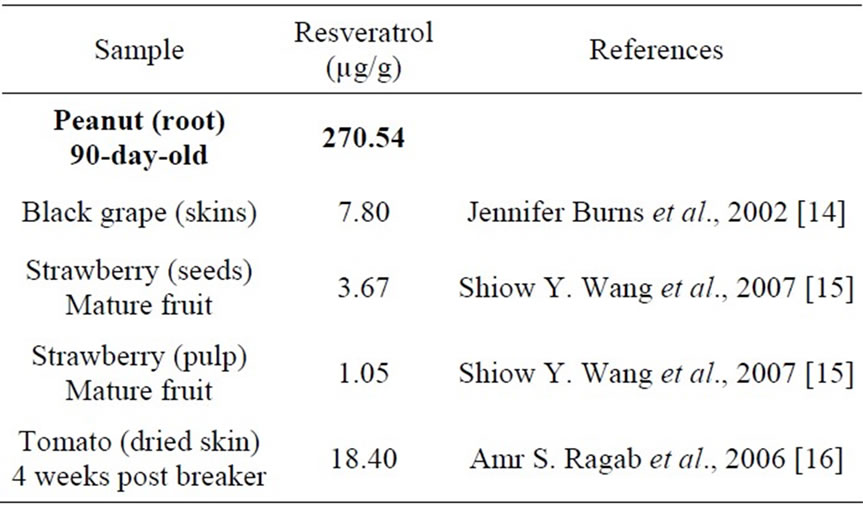
Table 4. Comparison of the content of resveratrol in Arachis hypogea L. with those of some other fruits.
in Table 4 elucidated that peanut root is the most potential source for resveratrol production. It seems in contrast to the common belief that resveratrol is produced only from grape and a very limited number of other edible species [14]. It provides us another new source to isolate resveratrol beside grapes.
5. Conclusion
From the roots of Arachis hypogea, we prepared four extracts and seven pure compounds. These extracts and resveratrol were tested for the antioxidant activity and got auspicious result. It encourages us to continue studying more deeply on the chemical constituents and biological activities not only of the roots but also of other parts of Arachis hypogea in order to use this plant more effectively.