Hydrothermal Growth of Zinc Oxide Nanorods and Glucose-Sensor Application ()
1. Introduction
Zinc oxide ZnO nanorods have attracted the increasing attention because of their wide variety of electronic and photonic device applications as a wide band gap semiconductor. One of interesting applications is a UV LED made of n-ZnO and p-ZnO. Since it is difficult to form p-ZnO, it may be replaced by p-NiO as a p-type material in the pn diode. NiO is known to be a p-type wide band gap semiconductor [1], and an ultraviolet-detector was made of a p-NiO/n-ZnO [2]. However, it is expected to improve the diode characteristic by using ZnO nanorods, which usually have crystallinity better than ZnO thin films because of the 1D growth. Utilizing the effect of substrate, ZnO nanorods can be formed selectively on the engineered substrate by the hydrothermal growth technique.
In addition, ZnO nanorods have great application potential in the field of biosensors due to their excellent biocompatibility, optical property, non-toxicity, chemical and electrochemical stability, high electron communication features and large specific surface area. The currently available sensors are based on electrochemical principles where the enzyme glucose oxidase serves as a molecular recognition element. Glucose can be converted in hydrogen peroxide and gluconic acid under oxygen consumption catalyzed by glucose oxidase. Hence, enzymetic electrochemical/amperometric glucose sensors based on glucose oxidase (GOx), have played a leading role in blood sugar testing. The GOx, is a widely used analytical enzyme for glucose detection. In spite of the many impressive advances in the design and use of enzymatic glucose biosensors, yet, the promise of reliable and accurate glucose sensing has not been fulfilled. There are still major challenges in achieving a stable, clinically accurate glucose monitoring. Many attempts have been made to fabricate glucose biosensors using ZnO [3-7] nanowires, nanorods and nanoparticles etc. to estimate glucose concentrations in human blood. Most of them are based on enzymatic and electrochemical techniques. However, these sensors are limited to their calibration range, biosensor response time, lifetime, stability etc. Luminescence quenching is a phenomenon that when the molecules are attached to the surface of the host material, the luminescence intensity of the host material decreases with the increased concentration of the immobilized molecules [6,7]. The luminescence quenching is usually understood in terms of the electron transfer reaction from the photo-excited particles to electron absorbing acceptors. It suggests that electron or hole acceptors adsorbed at the surface of nanostructures can change their luminescence properties and quench the exciton emission by fast electron transfer. We report non-enzymatic glucose sensing by PL emission quenching technique in ZnO nanorods and discuss their potential application as a glucose biosensor using β-D-glucose.
2. Experimental
Aligned ZnO nanorods were grown on GaN substrates with an area of about 1.0 cm2 by the hydrothermal growth technique using a mixture of 10 mM, Zn (NO3)2·6H2O and (CH2)6N4. The details of the growth have been described elsewhere [8]. Aqueous solutions of the β-D glucose (β form of dextrose, mol. wt. 180.16 g, 99% pure) purchased from Merck, were prepared in the concentration range of 0.25 mM to 40 mM, and an amount of 20 μL taken from each solution was drop casted on the ZnO nanorods to immobilize the glucose on the ZnO nanorod surface. The morphology and structure of ZnO Nanorods were studied by scanning electron microscopy (SEM) and X-ray diffraction (XRD) measurement. The photoluminescence (PL) spectra of the ZnO nanorods without glucose and with various concentrations of glucose were collected at 300 K using an Oriel PL system (SPEC-TRACQ2) with a single monochrometer and PMT detector. A wavelength of 250 nm was selected for excitation with a single monochromator from the spectrum of a Hg-Xe lamp.
3. Results and Discussion
Figure 1(a) shows high density, well aligned ZnO nanorods vertically grown on GaN substrate. The ZnO nanorods deposited on GaN were oriented along the (002) direction of the hexagonal lattice, as shown in Figure 1(b).
 (a)
(a)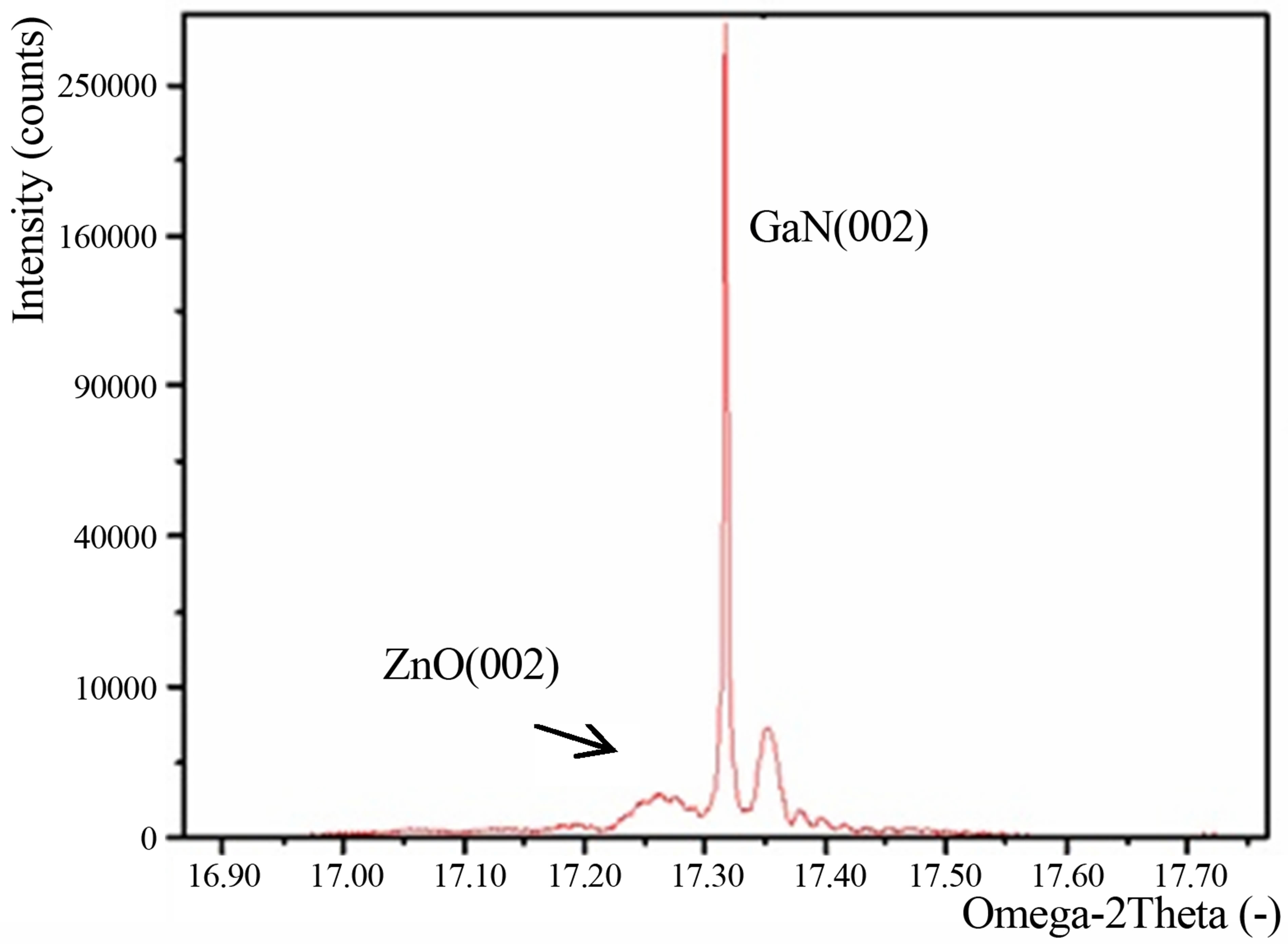 (b)
(b)
Figure 1. (a) SEM image and (b) XRD pattern of ZnO nanorods grown on GaN.
The photoresist on GaN was patterned to make an array of holes on a GaN-on-sapphire substrate by e-beam lithography, and then the hydrothermal growth of ZnO nanorods was carried out. More than one nanorod was grown when the hole size is 700 nm × 700 nm, while only one single nanorod was grown in a hole when the hole size decreased to 200 nm × 200 nm. However, they were not grown in all holes after 1 hr growth, as shown in Figure 2(a). A single nanorod was successfully grown in every hole of the sample by decreasing the concentration to 5 mM and increasing the growth time to 16 hrs, as shown in Figure 2(b). It is also noted that a lower concentration increases the aspect ratio.
Figure 3(a) shows the PL spectra of ZnO nanorods on GaN treated with the glucose of various concentrations in the UV wavelength range. The intensity is normalized with respect to the peak intensity of the as-grown ZnO nanorods. The PL spectrum of the as-grown ZnO nano-
 (a)
(a) (b)
(b)
Figure 2. Selective epitaxial growth of ZnO nanorods on the photoresist-patterned GaN. (a)1 hr, 10 mM; 16 hrs, 5 mM.
rods consists of two peaks, one in the UV range and the other in the visible wavelength region [8]. The narrower PL peak at 377 nm is associated with the near bandgap emission, while the broader peak at 580 nm is associated with point defects. Since the peak position and intensity vary in the visible wavelength region among the ZnO nanorod samples, but less in the UV region, we studied the quenching of PL in the UV region by the glucose attachment.
As seen in the figure, the PL peak intensity of the ZnO nanorods decreases with the increased glucose concentration without much change in the peak wavelength, and



Figure 3. (a) Photoluminescence spectra of ZnO nanorods treated with glucose of various concentrations; (b) shows the PL peak intensity of ZnO nanorods as a function of the glucose concentration, and (c) is the calibration plot obtained from (b).
does not further decrease for concentrations higher than 30 mM. A slight red shift in the peak is also observed with the increased concentration for concentrations of 30 mM or higher. Note that the PL rapidly quenches during the initial stage, and this was followed by a gradual decrease with the increased glucose concentration.
Figure 3(b) shows the PL peak intensity for various glucose concentrations, and Figure 3(c) is obtained from Figure 3(b) as a calibration plot. A good linearity is obtained in the glucose concentration range of 0.5 mM to 30 mM, corresponding to 9 - 540 mg/dL, as shown in the calibration plot. The sensor sensitivity, which is defined as the slope of the line, is found to be 1.4%/mM with a correlation coefficient of 0.99. The sensing range of the sensor is well suitable for a glucose sensor of human blood.
Several possible mechanisms for the PL quenching have been proposed. The surface reaction with a quencher may introduce the nonradiative surface defects. The charge transfer from a radiative material to a quencher was also proposed to be the main mechanism of PL quenching in many studies. Kim et al. recently observed the PL quenching of enzyme-conjugated ZnO nanocrystals treated with glucose and proposed H2O2 as a quencher [9]. The PL quenching occurred when the radiative transition of the excited electrons were suppressed by a quencher. The energies of the valence band and conduction band edges increased by the quantum confinement facilitated the electron transfer from the ZnO to H2O2. Since the amount of the PL quenching was proportional to the H2O2 concentration, which was also proportional to the glucose concentration, the PL quenching could be used as a measure of the glucose concentration. The PL quenching observed in Figure 3(a) is similar to that of the enzyme-conjugated ZnO nanoparticles. However, as mentioned in the Introduction, in the enzymatic sensor, the glucose oxidase is needed as a catalyst to oxidize glucose and produce gluconic acid and H2O2. In our case, the glucose oxidase is absent, and no catalysis is present to oxidize the glucose. ZnO nanorods themselves act as a catalyst to oxidize the glucose.
4. Conclusion
One single nanorod was successfully grown by the hydrothermal growth technique in every hole of the photoresist-patterned GaN when the hole size was 200 nm × 200 nm or less and the solution concentration was reduced to 5 mM. The ZnO nanorods grown on GaN by the hydrothermal growth technique show strong near band-edge PL, which is quenched by immobilizing glucose on the surface of ZnO nanorods. The amount of the decrease in the PL peak intensity increases with the increased glucose concentrations, and the calibration curve shows a good linearity with a sensor sensitivity of 1.4%/mM over the wide range of 0.5 - 30 mM, corresponding to 9 - 540 mg/dL. A high sensitivity and good lineality over the wide range make the use of ZnO nanorods highly favorable for a glucose-biosensor application. Furhtermore, the ZnO nanorod glucose sensor does not require an oxidase to oxidize glucose, because the ZnO nanorods themselves act as photocatalyses.
5. Acknowledgements
This project was in part supported by the Indo–JSPS project (DST-JSPS Project No. DST/JAP/P-68/09). S. N. S greatly acknowledges the JSPS Postdoctoral Research Fellowship for Foreign Researchers.