1. Introduction
H2S is a colorless, highly flammable and toxic gas, which in low concentration has an offensive odor similar to that of rotten eggs. As the Threshold Limit Value (TLV) for H2S is 10 ppm, there is a need for its detection in either ppm or sub ppm level [1-3]. α-Fe2O3, an intrinsically n-type semiconductor has been widely exploited as a gas sensitive material owing to its good structural and thermodynamical stability along with resistance to photocorrosion. In the present work, H2S sensing characteristics of Fe2O3 in both thin film and conventional forms have been investigated and compared. Our results indicate that thin film based sensors exhibited better response towards H2S in comparison to that of pellets.
2. Experimental
Fe2O3 thin films were prepared in two steps. In the first step, Fe films with ~100 nm thickness were deposited onto pre-cleaned (0001) Al2O3 substrates using electron-beam evaporation under a base vacuum of ~1.3 × 10−4 Pa. A high purity (99.99%) Fe powder was cold pressed in the form of a pellet and used as source material. These films were subsequently subjected to thermal oxidation at 800˚C in an oxygen environment (O2 flow: 50 sccm) for 2 h. Bulk sensors in the form of pellets were prepared by cold pressing commercial Fe2O3 powder followed by sintering at 800˚C for 2 h. The response characteristics were recorded as a function of temperature, gas (H2S, C2H5OH, NH3, CH4, CO, CO2, NO, and Cl2) and their concentrations using a static setup as described elsewhere [4]. For this purpose, the sensor was mounted on a Pt-100 based heater assembly in a leaktight glass chamber (net volume: 500 ml). Electrical contacts were made by thermally depositing two Au pads (120 nm thick). A desired concentration of the test gas in the chamber was achieved by injecting a known quantity of the test gas using a micro-syringe. The sensor response was calculated using the relations:
 (1)
(1)
where Gg and Ga are conductance in the presence of test gas and air, respectively.
3. Results
Figure 1 shows the X-ray diffraction (XRD) pattern ob-
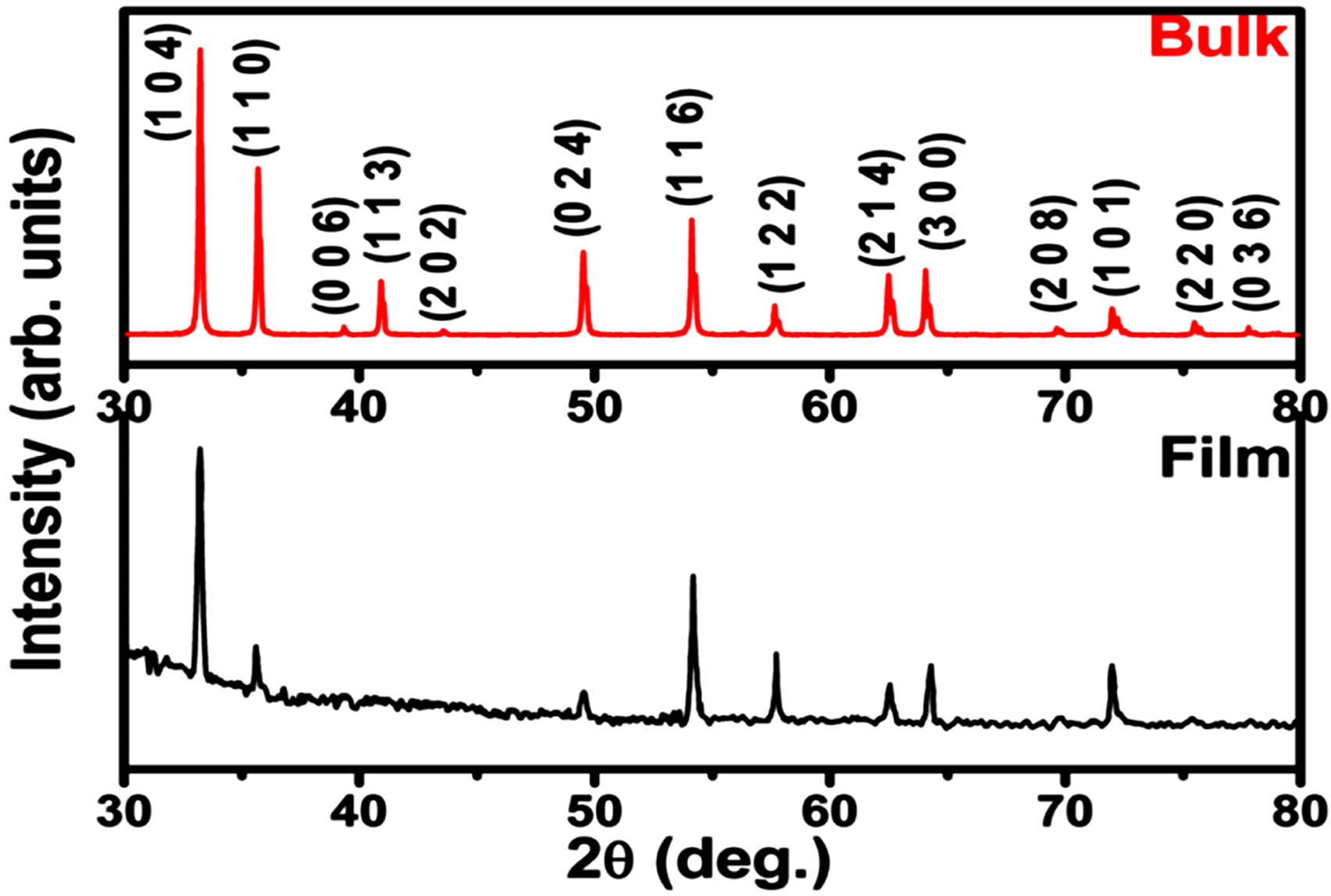
Figure 1. XRD diffraction pattern for Fe2O3 in bulk and thin film form.
tained for Fe2O3 thin film and bulk samples. All the peaks could be assigned to the single phase of Fe2O3 (hematite). The least-square fitting of the pattern indicated hexagonal rhombo-centred cubic unit cell structure with lattice parameters a = b = 5.028 Å, c =13.73 and α = β = 90˚, γ = 120˚, which are in agreement with the reported values [JCPDS card no. #79-0007].
Figure 2 is a plot of % response recorded for both the sensor films as a function of operating temperature towards 10 ppm of H2S. It is clearly evident that thin films and bulk sensors exhibit response maxima at 250˚C and 200˚C, respectively. At all the temperatures the response of thin films is higher than that of bulk samples. For instance, thin film sensors show, response maxima of 262% in comparison to 112%, exhibited by bulk samples towards 10 ppm H2S.
A Typical response curves for both the sensor films towards 10 ppm H2S is shown in Figure 3. The conductance of the sensors increases on exposure to H2S indicating an n-type response. Thin film sensors exhibited a maximum response towards H2S. A baseline drift was observed for bulk sensors.
Figure 4 shows the selectivity histogram recorded upon exposure to 10 ppm of various reducing and oxidizing test gases. It is clearly evident that both the sensors are highly selective towards H2S. A negligible response towards all the other interfering gases was observed.