Studies of lead toxicity on inflammatory damage and innate immune functions in testicular macrophages of male Swiss albino mice ()
1. INTRODUCTION
Exposure of animals to lead and its derivatives in day-to-day life are unavoidable due to its wide applications and usage. Lead poisoning is one of the oldest and the most widely studied occupational and environmental hazards [1]. Although lead is one of the most useful metals, it is also considered as one of the most toxic metals. It was indicated that lead can cause neurological, hematological, gastrointestinal, respiratory, reproductive, circulatory and immunological pathologies [2], as well as reproductive dysfunctions [3]. The effect of lead on immunocompetent cell activity has been well studied [4]. Role of macrophages in heavy metal induced immunotoxicologic effect has been reported earlier [5]. In short it affects adversely almost all the systems of the body.
Reproductive consequences of lead exposure are widespread [2], affecting almost all aspects of reproduction [6, 7]. Lead induces decreased sperm count, motility and increased morphological abnormalities in animals [8,9].
Lead is known to impede the male reproductive function; however the mechanisms through which the adverse effects are mediated are not clearly elucidated [10]. Lead intoxication results in the inhibition of testicular, epididymal and seminal vesicle function, altering the biochemical composition of this organ and affecting the normal development of germinal cells [11]. Growth effects of lead have been shown to be due to delay in the development of sex specific pituitary growth hormone secretion patterns [12]. In recent years study of immune-infertility has gained impetus. As the testes are immune-privileged organs, the effect of lead toxicity on testicular innate immunity remains a pertinent question. The present study attempts to evaluate the immunomodulatory properties of testicular macrophage functions, morphology, alteration of enzyme release, and pro-inflammatory response from murine testicular macrophages due to lead exposure in lead-intoxicated male albino mice.
2. MATERIALS AND METHODOLOGY
2.1. Animals
Adult male Swiss albino mice (average body wt 20 g) were divided into two groups 1) Control 2) Lead treated groups. The second group was injected (i.p) with lead acetate solution (10 mg/kg body wt) and the control group with 0.9% isotonic saline daily for 15 days [5]. The animals were kept in plastic cages in the departmental animal house. Animal care and protocols were in accordance with and approved by the institutional animal ethics committee. These animals were kept in an environment with controlled temperature (25˚C), humidity (45% - 50%), and photoperiod (12:12-h light-dark cycle). All the animals were fed standard diet ad libitum and had free access to water. All experiments were conducted in triplicate.
2.2. Analysis of Lead Bioaccumulation by Atomic Absorption Spectrophotometer
The testes from treated and untreated group were allowed to dry at 120˚C until reaching a constant weight, concentrated nitric acid and hydrogen peroxide (1:1 v/v) (SD fine chemicals) was added. The digestion flasks were heated to 1300˚C until all the materials were dissolved and diluted with double distilled water appropriately. The element lead was assayed using Shimadzu AA 6200 Atomic Absorption Spectrophotometer at the Sophisticated Analytical Instrument Facility (SAIF), NEHU, Shillong, Meghalaya. The results were expressed as µl/ml.
2.3. Isolation of Testicular Macrophages
Testicular macrophages were isolated following a slightly modified procedure of Sikorski’s method [13]. Macrophages from both control and lead exposed mice were used for assays.
2.4. Preparation of Bacteria (Staphylococcus aureus MC524) for Intracellular Killing and Phagocytosis Assay
To obtain bacteria in the mid-logarithmic phase 100 μl of an overnight culture made in nutrient broth was added to 10 ml of nutrient broth and incubated for 2 - 5 h at 37˚C with orbital shaking. The bacteria were washed in 10 mM sodium phosphate buffer (pH 7.4) and their concentration was estimated by spectrophotometry at A620 on the basis of the relationship: A620 0.2 = 5 × 107/ml [14].
2.5. Morphological Alteration
Testicular macrophages were taken in HBSS-BSA and fixed in 2.5% glutaraldehyde, centrifuged and the pellet resuspended in HBSS. Smears were drawn on glass slides and stained with Giemsa and observed under oil immersion microscope. Any cell deviating from spherical outline was scored as polarized and was expressed as a percentage of the total number of cells counted [15].
2.6. Scanning Electron Microscopy
The tissues were observed using a JSM-6360 (Jeol) SEM at the Sophisticated Analytical Instrument Facility (SAIF), North-Eastern Hill University (NEHU), Shillong, Meghalalya, India [16,17].
2.7. Phagocytosis
Testicular macrophage was taken on glass slides. Nonadherent cells were washed out with DPBS. Sheep erythrocytes (sRBC) is added to the glass slides with adhered macrophages, incubated and washed with DPBS. Slides were fixed in methanol and stained Giemsa and observed under oil immersion microscope. Phagocytosis index was calculated sRBC (average no. of SRBC per macrophage × 100) [18].
2.8. Intracellular Killing
Live bacteria incubated with testicular macrophage with DPBS-BSA. Non-ingested bacteria were removed by centrifugation. Cell containing ingested bacteria is resuspended in DPBS-BSA containing fetal calf serum. Samples are removed after different intervals and treated with gentamycin and plated in nutrient agar petriplate. Intra cellular killing was expressed as the percent decrease in the initial number of viable intracellular bacteria [19].
2.9. Myeloperoxidase (MPO) Release Assay
MPO release was estimated from macrophages following LPS stimulation in both control and treated mice using orthophenylenediamine (OPD) as substrate [20].
2.10. Nitric Oxide (NO) Release Assay
NO release was estimated after LPS stimulation in macrophages isolated from both control and treated mice with Griess reagent [21].
2.11. Cytokine Assay
Testicular cells were separated by density gradient centrifugation. Then testicular macrophages were obtained by adherence to plastic surface. A number of 1 × 105 viable cells in 0.2 ml RPMI 1640 medium supplemented with 5% FCS were distributed in microwells in flat 96 well microtitre plates and, after 24 h culture, supernatants were collected. Cytokine concentrations in culture supernatants were measured by sandwich ELISA estimating TNF-α using RayBio-Mouse TNF-α ELISA Kit. Biotinylated monoclonal secondary antibodies were used. The reaction was stopped with 3 M H2SO4 and the optical density of each well was measured in a 96-well plate reader at 450 nm. All determinations were done in triplicate. Standard curves were generated by recombinant mouse cytokines. Lower density limits were 10 pg/ml (TNF-α).
2.12. Statistical Analysis
The data were expressed as mean ± standard deviation. Data were analyzed using Student’s t-test (two-sample assuming unequal variances) for determining the significant changes over control values. The significance level was set at P < 0.05 and P < 0.001.
3. RESULTS
3.1. Concentration of Lead (ppb) in Testes of Treated and Untreated Group of Mice
After lead intoxication, the accumulation of lead was observed in testes of treated group whereas in control group traces of lead were not detected (Table 1).
3.2. Effect of Lead on Morphological Alteration of Testicular Macrophages
Morphological alteration was found to increase from 18.7% ± 1.19% in control to 77.5% ± 1.85% (P < 0.001) in lead treated group (Figure 1).
3.3. Effect of Lead on Morphology of Lead Intoxicated Testicular Macrophages by Scanning Electron Microscopy
The scanning electron micrograph of testicular macrophages isolated from the respective groups showed that in lead intoxication, testicular macrophages showed less differentiation and so, were less efficient in dendritic morphology (Plate 1(b)) as compared to control groups (Plate 1(a)), showing presence of psuedopods.

Table 1. Concentration of lead accumulated in testes of treated and untreated group.

Figure 1. In-vivo study of effect of lead on the morphology of testicular macrophages in adult male Swiss albino mice. (Mean ± S.D., P < 0.001).
3.4. Effect of Lead on Phagocytic Capacity of Lead Intoxicated Testicular Macrophages
In order to determine whether there was any alteration in phagocytic capacity of testicular macrophages due to lead treatment, the phagocytosis of heat killed S. aureus by macrophages was assayed. Result shows that lead causes a marked decrease in the phagocytic index from 26333.33 ± 1452.97 in control to 6666.67 ± 666.67 after lead treatment (Figure 2; P < 0.001).
3.5. Effect of Lead on Killing Capacity of Testicular Macrophages
This assay was performed to determine the killing capacity of intracellular S. aureus in cadmium treated and control group of mice. The result shows that lead treated mice are prone to infection and less effective in clearing invading pathogens as it was evident that testicular macrophages from lead exposed group were not able to kill the intracellular Staphylococcus aureus competently as shown in Figure 3; (P < 0.001).
3.6. Effect of Lead on Myeloperoxidase Release of Testicular Macrophages Isolated from Lead Intoxicated Male Swiss Albino Mice
Activation of macrophages with bacterial cell wall lipopolysaccharide (LPS) begins to express high levels of myeloperoxidase (MPO) enzyme. MPO decreases the free radical levels in our system. MPO release assay was performed to evaluate the effect of lead exposure on the release of this enzyme after LPS stimulation. Significant decrease in MPO released (µM) was observed. MPO released from the control group with LPS stimulation, showed a value of 72.9 ± 10.4 µM and 15.9 ± 2.23 µM in the lead treated group (Figure 4; P < 0.05).
 (a)(b)
(a)(b)
Plate 1.Effect of lead on macrophage differentiation. (a) The micrograph of normal macrophages at ×5500 and the bar is 2 µm; (b) Micrograph of lead treated macrophages at ×3700 and the bar is 5 µm.

Figure 2. In-vivo study of effect of lead on phagocytic capacity of lead intoxicated testicular macrophages in adult male Swiss albino mice. (Mean ± S.D., P < 0.001).

Figure 3. In-vivo study of effect of lead on killing capacity of testicular macrophages isolated from lead intoxicated adult male Swiss albino mice. (Mean ± S.D., P < 0.01).
3.7. Effect of Lead on Nitric Oxide (NO) Release of Testicular Macrophages Isolated from Lead Intoxicated Male Swiss Albino Mice
Macrophages, when activated with bacterial LPS, begin

Figure 4. In-vivo study of effect of lead on myeloperoxidase enzyme release in testicular macrophages isolated from lead intoxicated adult male Swiss albino mice. (Mean ± S.D., P < 0.05).
to express high levels of nitric oxide synthase which oxidizes L-argininine to yield citrulline and nitric oxide (NO). NO itself has potent antimicrobial acitivity and it can also combine with superoxide anion to yield even more potent antimicrobial substances. The effect of lead on NO release seeks to demonstrate the immuno-modulatory effect of lead. Significant decrease in NO released (µM) was observed in lead intoxicated group of mice. Result shows that lead causes a marked decrease in nitric oxide release (blue-control; pink-treated) from control 6.2 ± 1.45 µM to 3.6 ± 1.9 µM after lead treatment (Figure 5; P < 0.05).
3.8. Effect of Lead on Cytokines Pro-Inflammatory Release (TNF-α) of Testicular Macrophages Isolated from Lead Intoxicated Male Swiss Albino Mice
Intraperitoneal administration of lead in male Swiss albino mice led to an increase in the levels of pro-inflammatory cytokine; TNF-α from 195.33 ± 0.88 Pg/ml to 252 ± 1.73 Pg/ml (Figure 6; P < 0.05).
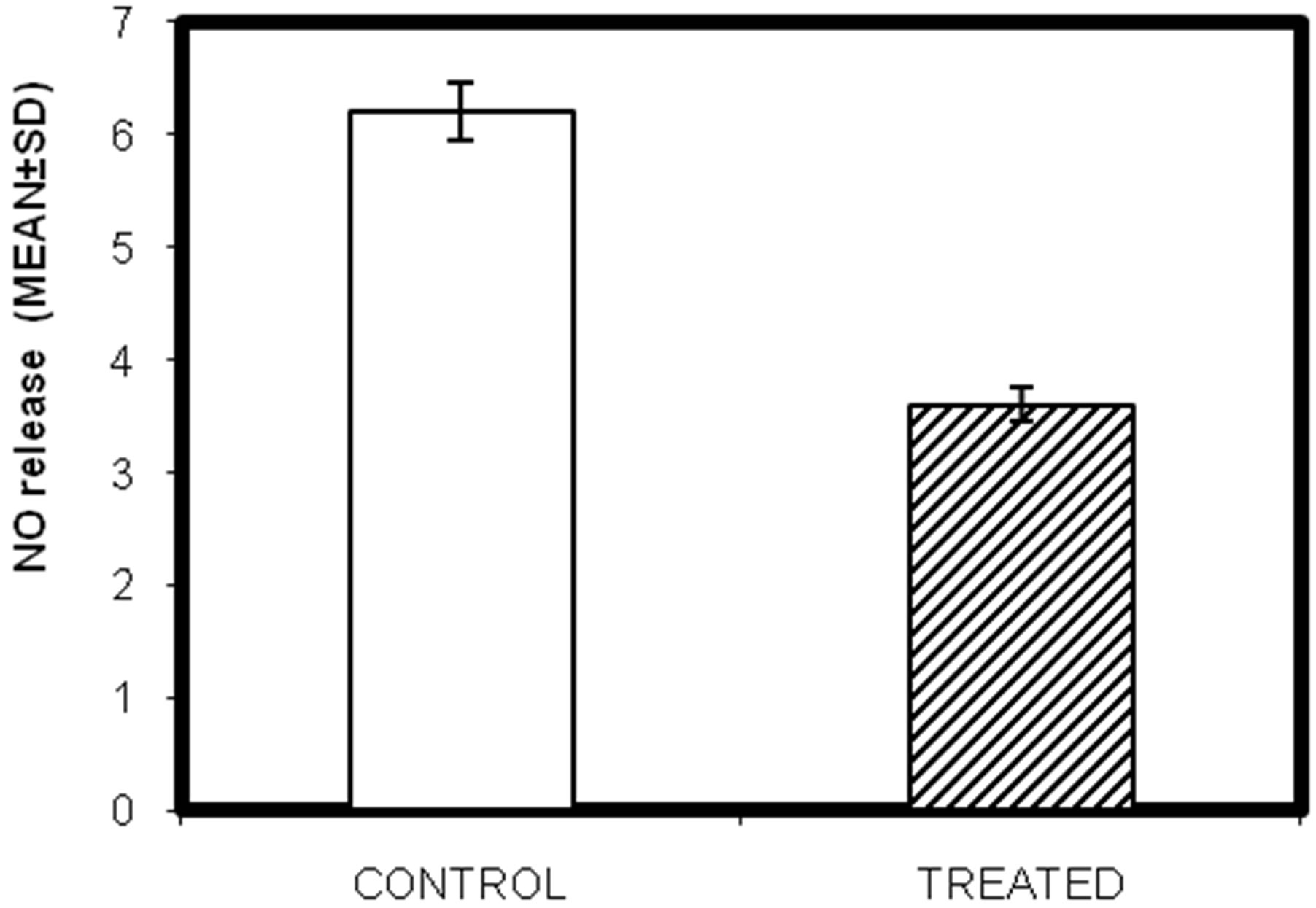
Figure 5. In-vivo study of effect of lead on nitric acid release in testicular macrophages isolated from lead intoxicated adult male Swiss albino mice. (Mean ± S.D., P < 0.05).

Figure 6. In-vivo study of effect of lead on pro-inflammatory (TNF-α) cytokine release in testicular macrophages isolated from lead intoxicated adult male Swiss albino mice. (Mean ± S.D., P < 0.05).
4. DISCUSSION
Lead is a heavy metal that can be toxic when introduced into the human and animal bodies by ingestion on inhalation. It causes various destructive effects [22]. In human, increased level of lead causes many serious diseases and dysfunction of organs [23,24]. Although the testicular macrophages have been shown to be involved in endocrinological mechanisms within the testis, little information is available concerning their immunological competence.
To demonstrate the effects of lead exposure on the number of differentiated macrophages, the macrophage cells deviating from greater to lesser spherical outline were measured and counted under a microscope (Nikon). From this investigation, it was found that lead treatment increases the number of undifferentiated macrophages. Lead impairs differentiation features of murine testicular macrophages. This may lead to deleterious effects in lead treated patients. This alteration is possibly associated with a marked reorganization of actin cytoskeleton. For further clarity scanning electon microscopy were performed. Scanning electron micrograph of randomly selected area of lead intoxicated testes showed much smooth periphery which renders the differentiation of testicular macrophages and was deficient of pseudopods (dendritic extension) (Plate 1(b)) as compared to the control where distinct dendritic extension was visible (Plate 1(a)). It means that the number of differentiated macrophages was reduced in lead treated as compared to control group which confirms the alteration in surface morphology of the macrophages.
The purpose of this investigation was mainly to determine if these cells were capable of performing two major functions after intoxication of lead: phagocytosis and killing of bacteria by opsonization and receptor-mediated phagocytosis as well as secretion of enzymes and cytokines.
Testicular macrophages were found to be very effective in phagocytizing and killing a gram-positive pathogenic bacterium Staphylococcus aureus but it was found that the phagocytic index and the intracellular capacity of the testicular macrophages were reduced significantly due to lead exposure. This study indicates that lead exposed mice are more prone to infection since the testicular macrophages are unable to clear invading microorganism as evident from reduced phagocytic activity. Priming of testicular by S. aureus may activate the cells and is more active to phagocytose. Prolonged exposure to lead also destroys the ingestion capacity of testicular macrophages as it can be obtained from the intracellular killing assay.
Exposure of organisms to bacterial infection results in secretion of various lysosomal proteolytic enzymes, and immune-effectors from the macrophages viz myeloperoxidase (MPO) and nitric oxide (NO) respectively are able to kill tumor and bacterial cells. Immature macrophages have lesser lysosomal content and are thus, less capable of secreting such enzymes. In the present study it is observed that MPO and NO release in lead treated group are significantly lesser than the control group (invivo). The immune effector NO plays a major role not only as a cell signaling molecule level but also as a potent bactericidal agent.
Testicular expression of the proinflammatory cytokines TNF-α was considerably enhanced with lead exposure. The pro-inflammatory cytokine TNF-α in the testis is produced by round spermatids, pachytene spermatocytes, and testicular macrophages. The type 1 TNF receptor has been found on Sertoli and Leydig cells and numerous studies suggest a paracrine mode of action for TNF-α in the normal testis. Our study reveals that release of TNF-α decreases with the treatment lead treatment.
The current study demonstrates that exposure of male mice to lead acetate resulted in alteration in morphology of testicular macrophages; reduced phagocytosis index of testicular macrophages indicates that lead treated groups are more prone to infection, as they cannot phagocytose efficiently and so cannot clear out the invading microorganism. The results of this study strongly suggest that exposure to the heavy metal lead in the form of lead acetate had severe effects on testicular macrophages. Hence, it can be summarized that the toxic potential of lead is overtly manifested in the testes and this may bear particular significance in heavy metal induced infertility.