Iatrogenic opioid dependence is endemic and legal: Genetic addiction risk score (GARS) with electrotherapy a paradigm shift in pain treatment programs ()
1. INTRODUCTION
1.1. Opioid Prescription Endemic
The extent of the opioid prescription epidemic in the USA was revealed in a weekly Morbidity and Mortality Report from the Center for Disease Control entitled “Prescription Drug Overdoses—a US Epidemic” at the beginning of 2012. According to the report there were about 27,000 unintentional iatrogenic drug overdose deaths that occurred in 2007 in the United States [1]. Over the past 10 years in an attempt to treat patient pain better, practitioners have increased the use of prescription opioid analgesics >600% [2]. By 2010, opioid analgesics, such as hydrocodone, methadone and oxycodone, were involved in three quarters (16,651) pharmaceutical overdose deaths [3]. Five million out of the nine million people, who use opioids long term [3], report use without medical need or a prescription (non-medical use in the past month) [4].
The breakdown of prescription overdoses is as follows: 1) 80% of all opioids are prescribed by a single clinician low doses (<100 mg morphine equivalent dose per day) [5,6], accounting for about 20% of all prescription drug overdoses; 2) 10% of patients are prescribed high doses (≥100 mg morphine equivalent dose per day) by single physicians accounting for an estimated 40% of prescription opioid overdoses [2,7] and 3) 10% of patients seek care from multiple clinicians and are prescribed high daily doses, accounting for another 40% of opioid overdoses [8].
These data suggest that prevention of opioid overdose deaths should focus on strategies that target a reduction in the availability of opioid medications. Electronic medical records have been initiated, and laws enacted to curb those who seek care from multiple doctors receive high doses, and are likely to be involved in drug diversion. Other approaches that could prevent these opioid overdoses and the morbidity that accompanies opioid dependence are the focuses of this paper. They are genetic testing for addiction liability and an alternative to narcotic analgesic treatment for pain. An alternative to pharmaceutical pain treatment is electrotherapy.
1.2. Characteristics of Electrotherapy
The use of electrotherapy for neuropathic pain is wellknown [9]. One device and program known as H-wave® electrical device stimulation (HWDS) is used clinically to expedite recovery from soft tissue injuries. A number of published studies have shown that HWDS produces a physiological response to tissue injuries and neuropathies [9-27]. Research that involves both animal models and humans has indicated that HWDS affects tissue healing in a positive manner and has analgesic properties. HWDS utilizes direct current electrical stimulation of the skeletal muscle surface. Low voltages and low stimulation frequencies are employed in this therapy.
A number of physiological mechanisms of action (MOA) of HWDS have been investigated recently in animals. Certainty of these mechanisms may have profound effects on the overall elements related to the healing process. The physiological responses to HWDS resulting in improvements, in tissue circulation, are due to the following MOA:
1) Muscle contraction of smooth and skeletal muscle (red, slow twitch) fibers via low frequency (1 - 2 Hz) stimulation causes loading of tissue while maintaining the low muscle force tension characteristic of being nontetanizing and non-fatiguing [10].
2) Arteriolar vasodilation accompanies HWDS and is due to a nitric-oxide mechanism demonstrated in rat studies [12].
3) Increase in new blood vessels angiogenesis following repetitive stimulation demonstrated bromouridine staining in rats [14].
4) Small smooth muscle fibers within the lymphatic vessels are directly and specifically stimulated ultimately leading to fluid shifts that reduce edema and protein clearance [22].
1.3. Tissue Loading Effect on Cellular Matrix and Genetic Expression
An important concept in orthopedic medicine and as such healing of tissue, especially bone, fibrous and skeletal muscle, is the acceleration of healing through loading [28,29]. It is noteworthy that even now when sophisticated new approaches such as the use of cytokines, cell transplants, and gene therapy have shown promise, none offer greater benefits than the loading of healing tissues [30]. Mak et al. [27] in a detailed review, have suggested that (once the injury reaches stability) loading may promote healing through a number of mechanisms involved in the healing process as it specifically relates to endurance and muscle recovery. Certainly the importance of loading on bone, fibrous tissue and muscle healing has been the subject of controversy since Nicholas Andre’ and Locus-Championniere, taught that early controlled activity promotes healing and restores functionality [30]. Other authorities argued against this view and suggested that absolute rest allows healing to proceed at the maximum pace and that loading may cause harm and inflammation thereby disrupting the natural healing process [31]. However, even as far back as 1892, Julius Wolf suggested that loading of bone may indeed cause changes in their structure, according to mathematical principles described as “Wolff’s law” [31].
“Recent investigations have provided insight into the mechanisms responsible for Wolff’s law and have shown that connective tissues other than bone also respond to changes in loading. These studies have shown that tissue-loading influences cell shape, gene expression, and synthesizing and proliferating functions. Bone, tendon, ligament, and joint capsule respond initially to loading by a mechanism of cellular detection of tissue strains, (these are) followed by modification of the tissue” [31].
Briefly, it is well known that the cells may detect tissue tension directly, or indirectly, through alterations in the cellular matrix, through deformation of the tissue. It is especially compelling that stretching or compression of mesenchymal cells alters the alignment of the cytoskeletal elements. These changes in cell shape induce gene expression and changes synthesis of matrix molecules and prostaglandins [31-40]. Eliasson et al. [40] evaluated gene expression following mechanical loading in rat Achilles tendon and found that, during the healing process, specific genes such as TNF-alpha transforming growth factor-beta 1 and procollagens 1 and 111 were down-regulated in loaded callus tissue at day three. However, by days 14 and 21, pro-collagen 1, cartilage oligomeric matrix protein, tenascin-C, tenomodulin, and scleraxis were all more expressed in loaded calluses. In the late phase of healing, tendon-specific genes (scleraxis and tenomodulin) were upregulated with loading, and the healing tissue might to some extent represent regeneration rather than a scar. Moreover, while intact tendons adapt slowly to changes in mechanical loading, the effect of mechanical loading on healing tendons or its absence is dramatic. The persistence of the response to a single episode of loading is unknown. The hypothesis that tissue has a “memory” of loading episodes, led to the idea that improved healing may be elicited by short loadings. Their animal findings indicated that information from a certain loading type and level once received by the tissue is “memorized” and the response lasts for many hours. This suggests that early short loading episodes might allow patients a better outcome following, for example, a ruptured Achilles tendon [41].
Healing back-pain through loading of the large muscle groups has been established long ago as the 1980’s [42]. The pathophysiology of the motion segment of the spine is influenced by several factors. Loading for various postures and exercises, including those at work has been successfully measured and delineated. The effect of various external factors has been measured, and nutriational pathways to the disc have been established. The body’s pain reducing-enkephalins are increased by activation of large muscle groups. Nachemson [41] suggests that early diagnosis and early mobilization of the patient should be of benefit, and that long term bed rest and inactivity must be prevented. Similar to others [30] Nachemson [43] in the 1970’s also believed that absolute rest was an essential component to the healing of back pain. A number of recent experiments have adequately resolved this controversy [31].
1.4. Cellular and Physiological Principles of Loading
It is well known that deformation of the cellular matrix can alter the macro-molecular organization, fluid flow (NO-dependent), streaming currents, pressure gradients, or electrical fields [40] and these matrix alterations certainly can influence cell function [40,44-48]. It is noteworthy that loading of a tissue can also alter the flow of nutrients and metabolites by affecting vascular perfusion and diffusion through the matrix [49,50]. The mechanisms of skeletal muscle response to changes in loading differ in some respects from those of bone and fibrous tissue because of the innervated muscle cells or myofibers, and to a lesser extent, an extracellular matrix [51]. Unlike connective-tissue cells, myofibers respond primarily to persistent changes in activity with alterations in cell structure, volume, and function. The importance here is that muscle cells respond to both passive stretching and active contraction [51]. There is no doubt that maintenance of normal muscle structure requires a minimal level of repetitive loading. Depending on variables such as age and gender, a low level of activity can lead to atrophy and decreases in muscle tissue organization and strength, while activity above the minimal level could lead to increases in muscle strength and mass [52].
We cautiously suggest that HWDS may be a valuable modality for the “loading” of bone to assist in the healing of osteoporosis and/or osteopenia related fractures. The importance of this potential is supported by public health and economic statistics about fractures from the National Institute of Arthritis and Musculoskeletal, and Skin Diseases [52] reported as follows:
“Osteoporosis is a major public health threat for 44 million Americans, 68 percent of whom are women; in the United States today, 10 million individuals already have osteoporosis and 34 million more have low bone mass, placing them at increased risk for this disease; one out of every two women and one in four men age 50 and older will have an osteoporosis-related fracture in their lifetime; more than 2 million American men suffer from osteoporosis, and millions more are at risk. Each year, 80,000 men have a hip fracture and one-third of these men die within a year; Osteoporosis can strike at any age; Osteoporosis is responsible for more than 1.5 million fractures annually, including approximately 300,000 hip fractures, 700,000 vertebral fractures, 250,000 wrist fractures, and more than 300,000 fractures at other sites; based on figures from hospitals and nursing homes, the estimated national direct expenditures for osteoporosis and related fractures total $14 billion each year” [53].
Interestingly, immobilization of a limb in a cast or traction for a prolonged period of time causes the rate of bone resorption to exceed that of bone formation [54,55] and subsequent loss of bone mass. Specifically, Goodship et al. [56] found that, without weight bearing, bone mass declines to less than half the normal value after 12 weeks. Conversely, persistent bone loading causes bone formation to exceed bone resorption and can result in increases in density, volume and strength [55,57]. This being the case it would be prudent to enhance treatment outcomes by understanding the risk for bone loss in the face of non-loading especially during immobilization periods are essential for normal skeletal growth and maintenance of skeletal structural and mechanical integrity relies on adequate Vitamin D and calcium. While reduced intake may result in osteoporosis and reduced bone mass, osteomalacia may be the result of severe and chronic vitamin D deficiency [58]. The vitamin D recaptor gene polymorphisms were investigated first, as possible determinants of bone mass loss and fracture risk, because of the importance of vitamin D in bone homeostasis [52]. Most of the epidemiological studies showed that the BAt haplotype, from BsmI, ApaI and TaqI polymorphisms in VDR, are associated with a lower bone mineral density in women and a higher risk of osteoporotic fractures. Knowledge of this risk may indicate that the level of loading may need to be adjusted up or down based on these gene polymorphisms [59]. There is also the likelihood that parathyroid levels may also play a significant role in bone resorption risk due to nonloading. Moreover, the plasma levels of Parathyroid Hormone have been tied to not only osteoporosis but to dementia and stress, which could also influence the bone-healing outcome [60].
Based on unpublished very preliminary information on only one patient, we found that H-Wave induced bone loading over a 4-month period increased bone density via X-ray. We must await additional subjects to establish H-Wave induced bone mass. In addition, in an unpublished review, a physician evaluation found that HWDS induced new bone growth seen on X-ray in another patient with a comminuted fracture of the tibia following 90 days of treatment. We do not know if, in this patient the healing process was so strong that new bone growth may have resulted without HWDS. The impetus to continue to evaluate HWDS in patients who have bone injury seems parsimonious.
It is noteworthy that, unlike other tissue, (with the exception of perhaps liver) bone is the only tissue that heals with its original tissue. In fact, bone as opposed to all other human tissue—especially tendon, muscle and ligaments—which heal, with scar tissue rather than the original tissue structure. Moreover, scar formation in these healing tissues can be modulated differently (qualitative and quantitative) depending on the healing environment and possibly genetics.
We hypothesize that potentially similar to muscle loading HWDS may reduce bone resorption via physiological responses and MOA. This is further supported by the suggestion of Burr et al. [52] that regular contraction of the muscles of an immobilized limb may decrease the rate of loss of bone.
Along similar lines, immobile dense fibrous tissues that normally resist (tendon, ligament, and joint capsule) alters matrix turnover, so that with time, matrix degradation exceeds formation. In terms of matrix metaloprotein rat studies reveal that in dental implants, localization and bone formation with osteocalcin were associated with high shear stress areas near the helical threads of loaded implants. The apical two-thirds of each unloaded bone implant showed fibrous tissue and extensive MMP-13 localization adjacent to the implant [58]. In agreement with estimated bone-implant contact ratios, between 14 and 21 days, a significantly higher contact ratio was demonstrated in the loaded group while a steady decrease in contact ratio occurred in the unloaded implant group [52]. Other studies by Akeson [59] that looked at limb immobilization, found that prolonged limb immobilization, especially after 6 weeks, may have increased collagen cross-linking and decreased collagen mass, decreased the glycosaminoglycan and water content, and changed the degree of orientation of the matrix collagen fibrils. Interestingly MRI studies have shown that HWDS causes beneficial fluid shifts as observed in magnetic resonance imaging studies which should impact this unwanted result [10].
Decreased loading also affects ligament, tendon and capsular insertions into bone. For example, decreased ligament loading due to immobilization usually produces more extensive changes in the periosteum type of insertion. With knee injuries prolonged immobilization causes bone resorption around the periphery of the cruciate ligament insertions, but only limited resorptive activity beneath the insertion site and in the zone of fibrocartilage [61]. In fact, in animal experiments it has been shown that immobilization after 6 - 8 weeks requires loading of the ligament insertion site for as long as one year to obtain healing [62]. Furthermore, repetitive loading can increase the strength, size, matrix organization, and collagen content of tendons, ligaments and their insertions into bones. In support of this statement Slack et al. [62] showed that tension to cultured tendons increased protein and DNA synthesis. Other studies revealed that intensive exercise training, in swine, augmented extensor tendon strength, collagen concentration and weight [63]. The same researchers also found that in vitro rabbits showed that localized increased loading alone causes adaptation of dense fibrous tissue [64].
1.5. Nitric Oxide and Muscle Activation
Nitric oxide (NO) known as the “endothelium-derived relaxing factor”, or “EDRF”, is biosynthesized endogenously and NO production plays a major role in the physiology of circulation [65,66]. Deficiency of this putative neurotransmitter has been linked to a number of diseases/disorders of the circulatory system [67]. NO is synthesized in normal muscle fibers by the neuronal NO and the endothelial NO isoforms of nitric oxide synthase NO. NO contributes to the regulation of several processes such as excitation-contraction coupling and mitochondrial respiration both very important in loading [68]. In experiments by Pye et al. [68] muscle fibers were isolated from the flexor digitorum brevis muscle of mice, and intracellular NO production was visualized in realtime using the fluorescent NO probe 4-amino-5-methylamino-2’,7’-difluorofluorescein diacetate (DAF-FM DA). Electrically stimulated contractions in isolated fibers increased the rate of change in DAF-FM fluorescence by approximately 48% compared to non-stimulated fibers (P < 0.05). The rate of change in DAF-FM fluorescence in the stimulated fibers returned to control values by 5 min after contractions. Treatment of isolated fibers with the NO synthase inhibitors NG-nitro-L-arginine methyl ester hydrochloride (L-NAME) or NGmonomethyl-L-arginine (L-NMMA) reduced the increase in DAF-FM fluorescence observed in response to contractions of untreated fibers. Since electrical stimulation causes an increase in muscle NO (during loading), it is feasible that the Smith et al. [13] study showing a NO dependent increase in microcirculation of the rat by HWDS together with the work of Pye et al. [68] may provide the MOA that can explain this observed phenomena.
The non-tetanizing and non-fatiguing properties of HWDS, unlike other electro-therapeutic devices and or drugs, may be most beneficial in augmenting skeletal muscle loading induced tissue blood flow linked to an increase in NO production during periods of healing [13,14]. It is noteworthy that there are a number of pharmaceuticals that induce a NO dependent vasodilatation, for the treatment of circulatory dysfunction; however, their positive therapeutic effects have been clouded by moderate to severe adverse reactions in patients [69]. The mechanism of such a benefit as HWDS induced NO production, may reside in the importance of NO in cell communication and the inflammatory process a necessary repair phenomena that should not be interrupted during early stages of tissue repair [69]. An example is that NO elicits functional MMP-13 protein tyrosine nitration during wound repair [70]. The beneficial effects of NO on tissue repair may be attributed to its functional influences on angiogenesis [22,71], inflammation, cell proliferation, matrix deposition, and remodeling [10,11, 72].
1.6. Angiogenesis and Musculoskeletal Healing
Angiogenesis, new blood vessel formation, takes place following a disturbance in the luminal side of blood vessel endothelium. Increased stretch/wall tension/shear stress initiated by increased blood flow velocity, capillary hematocrit or the release of NO, prostaglandins, growth factors or other humoral agents may damage luminal endothelium and/or their basement membrane [24,73]. Angiogenesis, is part of normal physiology examples including, female reproductive organs, skeletal and cardiac muscle due to increased training and as a response to hypoxia and increased altitude [73].
The mechanisms responsible for either the acute, shortterm or the long-term effects of HWDS on the microcirculation may involve angiogenesis [24]. In fact, in rat studies we have found that bromouridine staining demonstrated increased staining for new blood vessels in chronically stimulated hind limb of rats following HWDS [24]. We suggest that this induction of angiogenesis is due to the chronic vasodilatation associated with chronic loading of the muscle through non-tetanizing and nonfatiguing stimulation. Moreover, alterations in wall tension produced by vasodilation or changes in microvascular wall shear stress appear to be stimuli for elaboration of new blood vessel formation [71]. Hudlicka investigated these mechanisms.
Hudlicka’s [73] experiment exposed skeletal muscles to: 1) chronic electrical stimulations to increased activity; 2) various vasodilators to blood flow, long-term; 3) CoCl2 administration of to increase hematocrit also longterm; and 4) removal of agonist muscles effect long-term stretch. Electron microscopy with histochemical staining was used to visualize an increased capillary/fiber ratio, had occurred in every exposure but 3). An estimation of capillary proliferation was made by label indexing capillary-linked nuclei, for bromodeoxyuridine found capillary proliferation in 1). Capillary diameter increased, arterioles underwent transient widening and veinuoles did not change with chronic electrical stimulation. The velocity of red blood cells (Vrbc), capillary hematocrit, wall tension and calculated shear stress were increased compared to control muscles, by administration of the alpha 1-blocker prazosin. Longer sarcomeres caused initial concomitant stretch of capillaries in stretched muscles. Stretched muscles also caused decreased blood flow.
Hudlicka [73] showed that immunohistochemistry in stimulated or stretched muscles revealed increases in low molecular mass endothelial cell-stimulating angiogenic factor (ESAF) in 1), 2), and 4). There was no evidence of mRNA expression for the growth factor itself or basic fibroblast growth factor (bFGF). After administration of indomethacin or L-NNA, the involvement of NO and prostaglandins was demonstrated when BrdU was incurporated into capillary-linked nuclei in stimulated muscles. Thus, changes in the microcirculation leading to increased shear stress and/or capillary wall tension may, as observed in HWDS, induce proliferation of endothelial cells either directly, or by release of various humoral factors. This phenomenon is the subject of additional research (Figure 1).
1.7. Protein Clearance and Cytokines in the Healing Process
It is well known that, in healing, the tissue responses that can restore tissue integrity (structure and function) after injury result from complex humoral and specific vascular events [74]. Understanding the healing process, which includes inflammation, repair and remodeling, enables the clinician to treat the injury appropriately and systematically. The continuous process begins with the release of inflammatory mediators such as interleukin factors (regulated by polymorphisms of TNF-Alpha) and ends when remodeling of the repair tissue reaches a homeostatic state. It is at this time in the healing process that loading can positively affect tissue healing from the repair stage through the remodeling stage [75].
Multiple mechanisms are necessary to temporally restrict and direct the effects of potent mediators of immune reactions, inflammation, and tissue repair. Recent studies have implicated the protein alpha 2-macroglobulin (alpha 2M) as a mediator that regulates the activeity and distribution of many cytokines. These include platelet derived growth factor (PDGF), transforming growth factors-beta (TGFs-beta), tumor necrosis factoralpha (TNF-alpha), interleukin-6 (IL-6), inter-leukin-1 beta (IL-1 beta), nerve growth factor (NGF) and fibroblast growth factor (b-FGF) [75].
As early as 1985, Proctor et al. [76] suggested a change in the synthesis of one or several different cytokines might result in disease and represent either changes in their alpha 2M-receptor-mediated clearance/targeting mechanisms or in the production of alpha 2M “cytokine scavengers”. Moreover, the “sequence identity” between the alpha 2M Receptor and the LDL-receptor related protein and provides a theoretical basis for interference with cytokine clearance by association of competing lipoprotein ligands with this cytokine clearance pathway. In addition, activated alpha 2Ms or enhancement of alpha 2M-receptor-dependent cytokine clearance might be novel strategies for preventing the harmful systemic or local effects of excess cytokines such as TGF-beta s and TNF-alpha in vivo.
“Fibronectin” found, in connective tissues (in blood) and most basement membranes are a group of immunochemically related glycoproteins that have an adhesive function. Distinct binding domains serve to mediate between collagen, cells like macrophages, fibrinogen, and fibroblasts, and/or certain glycosaminoglycans like heaprin. The “mononuclear phagocytic” system is comprised of macrophages and monocytes phagocytic cells. The monocytes attachment to gelatin-coated surfaces and gelatin-coated RBCs or latex particles attach to surfacebound monocytes. This adhesion is mediated by plasma fibronectin (Cig).
Protein clearance, as a natural step in the healing process, is a very complex phenomena and is not simply the milking action of the smooth musculature of the lymphatics. Thus, the ability to clear out unwanted elements is not based on simple mechanics. It is indeed chemical in nature. The strong affinity between fibrin and fibronectin is and may provide a mechanism for the recognition of fibrin-fibrinogen complexes and their subsequent clearance from the blood, on mononuclear phagocytes attached to fibronectin receptors [76]. Fibronectin bound to denatured or exposed collagen at sites of injury may lead to macrophage attachment and differentiation and probably plays an important role in wound organization and healing [77].
Unpublished preliminary data from the laboratory of Thomas Smith at Wake Forest University School of

Figure 1. Illustrates loading of H-Wave coupling cellular mechanisms and genetic analysis.
Medicine (evidence in a few rats) suggest that HWDS induces protein clearance. Although, this may occur by the purely mechanical event of inducing contraction of lymphatic smooth muscle, it would be of interest to assess the role of HWDS in protein clearance and to determine fibronectin activity in injured tissue pre and post HWDS.
1.8. Clinical Implications
An examination of the literature has revealed that early controlled activity promotes healing of bone, tendon, ligament, and skeletal muscle. While there is more intensive research left to be done regarding the optimal application of controlled tissue loading and the effects of loading under these conditions, we cautiously suggest that the incorporation of HWDS certainly when the injured loci is stabilized should confer a positive healing outcome. There are a number of important clinical points that must be considered. In particular, loading of injured bone, tendon, ligament, and skeletal muscle should be minimized during the period of acute inflammation response that follows tissue injury. Variables include; the type of injury and type of tissue repair required the time from tissue inflammatory response and formation of the initial repair. Buckwalter and Grodzinsky [29] provide a schematic discussion of when to apply loading for different types of injury and tissue repair. Accordingly, they suggest that in a clinical setting, loading of healing tissues may be done with various combinations of both active and passive motion with or without any resistance, or even by isometric muscle contraction.
An important clinical aspect that should be considered herein, is that animal research has shown that repetitive HWDS induces significant angiogenesis compared with single or intermittent HWDS [11,13]. This finding supports the concept that tissue healing may be induced by adding tissue loading. As mentioned earlier, muscular contraction or shear-wall stress is the best-known factor for the intrinsic production of angiogenesis. Interestingly, by stimulating slow-twitch myofibrils, with associated mitochondria activity, a larger and denser network of new blood vessel formation or angiogenesis will occur. As stated earlier we would like to emphasize that although other electrotherapeutic devices can cause tissue loading due to muscle contraction using fast-twitch muscle fibers (e.g., TENS, EMS, NMES , High-volt galvanic, interferential [IF]), H-Wave® therapy utilizes slow twitch muscle fibers in a non-fatiguing contraction and because of this property has been shown to induce fluid shifts as determined by MRI studies [10,20] and the resultant enhanced microcirculation is dependent on NO mechanisms(s) [13].
Finally, these microvascular and hemodynamic findings coupled with the state of the art concept of the applicability of tissue loading provide an understanding of the mechanisms linked to increased perfusion, and tissue repair and remodeling. We suggest that following additional confirmatory research in both animals and humans evaluating HWDS effects on stress vs. strain (measurement of tension), measurements of loading (dynes) and stiffness, we have potentially achieved scientific advancement; that is an alternative to addicting pharmacological analgesics and toxic anti-inflammatory agents that could also induce loading with a concomitant increase in tissue healing. To reiterate, a meta-analysis [20] found a moderate to strong effect of the HWDS in providing pain relief, reducing the requirement for pain medication(s), and increasing functionality. The most robust effect was observed for improved functionality, suggesting that the H-Wave® device and program may facilitate a quicker return to work.
Most recently a double-blinded study involving H-wave pad-placement immediately following rotator cuff surgery used low frequency (tension control) gradually increases in intensity over the course of the experiment. This experiment yielded a very significant positive outcome especially as related to range of motion (ROM) and agrees with the potential for early muscle loading in wound healing [29]. HWDS compared to placebo induced a significant increase in ROM in positive management of rotator cuff reconstruction, supporting other previous research on HWDS and improvement in function. Interpretation of this preliminary investigation while suggestive of significant increases in ROM of Post-Operative Rotator Cuff Reconstruction, warrants further confirmation in a larger double-blinded sham controlled randomized study [21].
Interestingly, the ability to control loading tension by HWDS may provide an earlier intervention that will assist in tissue healing without interrupting the natural healing process as discussed by Buckwalter and Grodzinsky [29]. They described when to initiate muscle loading as follows:
“In some minor injuries and following certain surgical procedures, there is minimal acute inflammation, and the tissues are stable; in that situation, motion and loading can be initiated almost immediately. When there is minimal tissue loss or necrosis, little disruption of the blood supply, and close approximation of the surfaces of the injured tissues, the initial repair tissue forms within 3 to 10 days. Examples of these types of injuries include closed impacted or minimally displaced metaphyseal fractures, repaired flexor tendon lacerations, medial collateral ligament tears, and skeletal muscle strains. Motion and loading of these injured tissues can be started as soon as the acute inflammation and accompanying pain have subsided. In injuries with segmental tissue loss, compromised blood supply, or large gaps between tissue surfaces, formation of the initial repair tissue requires more time. Loading before stable repair tissue has formed may adversely affect the result. In the clinical setting, loading of healing tissues may be done with various combinations of active and passive motion, with or without resistance, or by isometric muscle contraction” [29].
There are known genetic anteceedents to addiction liability and as such we turn our attention to the important role of neurotransmitters especially dopamine and pain sensitivity.
1.9. Pharmacological of Pain Control
Opioids like heroin and morphine and psychostimulant drugs like amphetamine are pharmacologically effective in treatment of chronic pain. Interestingly, amphetamine and related drugs that can potentiate opioid analgesia and counter cognitive disturbances and opioid-related sedation are sometimes administered as an adjuvant analgesic to relieve cancer pain. Studies in rats have shown that psychostimulants potentiate the analgesic effect of morphine in an animal model of persistent pain support these clinical findings [78]. Evidence that sites rostral to the brainstem play a crucial role in the effects of opioid analgesics and psychostimulant drugs is increasing.
Opioids acting at sites in the brainstem and spinal sites can inhibit pain. They modulate the activity of the descending pathways of the brain stem that project onto the spinal cord. The periacqueductal gray of the brain stem is a primary site of action where opioid receptors are activated at the nucleus raphe magnus, by direct projections onto serotonin-containing cells. In turn, these cells activate neurons that project to the dorsal horns of the spinal cord, via the dorsolateral funiculus, where they inhibit cells that transmit information about noxious (painful) stimulation to supraspinal sites from the periphery. The role of the descending brainstem pain-suppression system plays, is larger, in the suppression of brief, rapidly rising, transient, and well-localized phasic pain, than it is in the suppression of injury-produced persistent inescapable tonic pain. However, several lines of evidence suggest that the inhibition of tonic pain requires the activation of dopaminergic neural systems in addition to those required to inhibit phasic pain [79,80].
1.10. Mesolimbic Dopamine in the Suppression of Tonic Pain
There is little information to date concerning the identity of the endogenous pain systems that serve to inhibit tonic pain. The suppression of tonic pain involves systems in addition to those known to suppress phasic pain, and that these systems appear to involve forebrain sites, rostral to the brainstem. A clue to this problem is that both opioids and psychostimulants reduce tonic pain and increase transmission in mesocorticolimbic dopamine neurons known to be activated by natural rewards such as food and sex. These neurons arise from dopamine cell bodies that lie in the ventral tegmental area (VTA) and project to various forebrain sites such as the nucleus accumbens (Nacc), amygdala, and prefrontal cortex. Opioids cause the release of dopamine from these neurons through their indirect activation (see reward cascade Figure 2), whereas psychostimulant drugs such as amphetamine and cocaine increase dopamine extracellularly by decreasing reuptake and/or inducing release. Moreover, opioids and psychostimulants have both rewarding effects and analgesic effects in the clinical setting, suggesting that reward and analgesia might share common neural substrates [81]. It was found that dopamine-depleting 6-hydroxydopamine lesions of the ventral midbrain, which contains the cell bodies of the neurons that give rise to ascending forebrain projections, block the analgesic effects of systemic morphine and amphetamine in the formalin, but not the tail-flick test. Their findings provided the first evidence that mesolimbic dopamine neurons play a role in the suppression of tonic, but not phasic pain. In recent studies, Taylor et al. [82], found that while the D1-selective agonist SKF 38393 was without effect at a dose of 0.5 nmol/side, the D2-selective agonist quinpirole dose dependency (0.05 - 5 onmol/side, bilateral) inhibited the persistent phase of formalin-induced nociception. This was blocked by pre-administration of a selective D2-dopaminergic antagonist raclopride. These results indicate dopamine agonists that activate D2 receptors in the Nacc, inhibit inflammatory pain.
In this cascade, stimulation of the serotonergic system in the hypothalamus leads to the stimulation of delta/mu receptors by serotonin to cause a release of enkephalins. Activation of the enkephalinergic system induces an inhibition of GABA transmission at the substantia nigra by enkephalin stimulation of mu receptors at GABA neurons. Both the endocannabinoid and glutamate receptors influence GABAergic activity. This inhibitory effect allows for the fine tuning of GABA activity. This provides the normal release of dopamine at the projected area of the NAc, a major reward site of the brain (Blum et al. 2013 with permission [83]).
1.11. Dopamine D2 Receptors and Chronic Pain
Changes in synaptic neurotransmission plasticity are thought to have a role in chronic pain. Animal studies suggest that dopaminergic systems in the striatum and cortex of the brain participate in pain modulation and transmission. In animal models for persistent pain, a role for dopamine D2 receptors has been reported in the mediation of pain inhibition by dopamine [84]. Hagelberg et al. [85] showed using positron emission tomography (PET) that hypodopaminergic function is associated with pain due to burning mouth syndrome. This effect involves mu receptor interaction [86]. Furthermore, increased D2 receptor availability possibly due to decreased[18F] FDOPA uptake, have been demonstrated in the putamen in, burning mouth syndrome. Furthermore, the increase in left putamen D2 receptor availability and the decrease in D1/D2 ratio imply that altered striatal dopamineric system as evaluated by PET may also be involved in chronic orofacial pain conditions [87]. Essentially, we hypothesize here that low neurological dopaminergic function may herold a predisposition to low pain tolerance. With support from current research for this concept, carriers of the D2 Taq A1 allele as seen in Reward Deficiency Syndrome (RDS) behaviors may need to enhance their dopamine release with a neuro nutrient D2 agonist to boost their pain tolerance [88].
1.12. Stress and Pain
The effects of excessive stress in modern life lead to chronic states of fatigue-related depression. According to the American Academy of Family Physicians, about 2/3 of all office visits are related to stress and depression. Numerous examples in the literature support the contention that an individual with chronic pain, is indeed in a stressful condition which increases neuronal firing. Furthermore, numerous studies have shown that if an individual has the DRD2A1 variant of the DRD2 gene, the dopamine D2 receptors reduction is associated with an inability to cope with stress [89-91]. D2 receptor messager RNA (mRNA) can also be reduced by stress, in the substania nigra the lateral part of the VTA, the basal ganglia, and especially in the NAc [92].
The concept that dopamine systems in the forebrain are involved in mediating the behavioral effects of mild chronic stress is supported in this work. Further, the view that in subjects with pain and resultant chronic mild to moderate stress, reduced D2 receptor site density, reduced mRNA message and enhanced catecholaminergic neuron firing frequency would be quite receptive to l-tyrosine, a dopamine precursor is also supported here. Moreover, it is known that neuronal dopamine depletion can induce an independent inhibitory state endproduct for tyrosine-hydroxylase, which will also respond to l-tyrosine supplementationn. In this regard, in order to provide an up-regulation in D2 receptors, we propose a natural and personalized solution designed to facilitate a gradual and constant release of dopamine, the effect of enhanced opioidergic activity due to d-phenylalanine (a known enkephalinase inhibitor) on substania nigra GABA neurons. The main point here is that pharmacological manipulation of up-regulation of dopaminergic pathways will ultimately lead to reduction of stress since it well known that the dopamine molecule is considered the endogenous anti-stress substance.
1.13. The Pathophysiology of Chronic Widespread Pain as Implied by Stress and Dopamine
The relationship between endorphins, stress, and hypothalmic-pituitary-adrenal (HPA) axis is well researched [93]. Certainly stress plays a critical role in both addiction and relapse. It is known that certain environmental and genetic elements play a significant role in dysregulation of brain reward pathways and drug dependency. In fact, polymorphisms of the dopamine D2 receptor have been associated with mechanisms for coping with stress and posttraumatic stress disorder [94]. Interestingly, stress can both exacerbate the pain or even induce a painful condition. Dopamine transmission in mesocorticolimbic dopamine neurons is activated by exposure to stress [95]. This effect in the VTA appears to involve opioid mechanisms. Specifically, intra-VTA infusions of naltrexone, the opioid receptor antagonist, prevent the stress-induced activation of dopamine metabolism in the prefrontal cortex and NAc and block stress-induced analgesia in the formalin test [79,80], while stress exposure, causes met-enkephalin release into the VTA [96]. This observation when combined with findings that indicate that tonic pain can be inhibited by exposure to stress and analgesia in the formalin test, is induced by intra-VTA morphine, suggest that a mechanism that underlies the stress-induced inhibition of tonic pain might be the endogenous release of opioids in the VTA. In addition, a similar role for the release of the tachykinin neuropeptide, substance P (SP), in the stress-induced suppression of tonic pain in the VTA has been proposed. Indeed, midbrain dopamine neurons activated by SP did inhibit tonic pain in the formalin test [79]. The current data suggest a release of SP in the VTA caused by exposure to stress induces analgesia which activates mesocorticolimbic dopamine neurons. Finally, the ability to increase dopamine release into the NAcc is shared by opioids, amphetamine and SP. Moreover, dopamine metabolism and extracellular levels of dopamine in the NAc are augmented by opioids administered systemically or into the VTA.
With that background, it follows that this hypothesis; that dopamine D2 activation can attenuate tonic pain, we embrace the concept of supportive research in the area of developing a natural method that can induce the preferential release of dopamine in mesocorticolimbic pathways. Thus support of an attenuation of stress has been found with a variant of a complex with dopaminergic activation properties shown in one double-blind placebo controlled study [97]. We further hypothesize herein that unless there is a way of increasing endogenous opioids, which in turn inhibit GABA causing dopamine release in the NAc, simple neurotransmitter precursors will not be as effective in reducing tonic pain.
1.14. Clinical Perspectives
Together with the current findings that HWDS or other similar electrotherapeutic devices bring about pain relief, increase tissue perfusion, blood flow, induce NO-dependent tissue microcirculation and angiogenesis (during repetitive stimulation), tissue loading should be included in a clinical approach to facilitate recovery from tissue injury.
Being cognizant that there are a variety of methods, including the use of cytokines, cell transplants, and gene therapy, to restore, repair and remodel injured tissue, the addition of a modality purported to cause loading in a non-fatiguing and non-tetanizing way focused at overcomeing the pain and loss of function associated with tissue injury should be considered.
Our review of this core of associated research provides reasonable rational to propose that when used appropriately, HWDS could improve tissue healing.
Recent advances in the field of muscle recovery and endurance have indicated that a number of interesting MOA are linked to the healing process. The newly accepted view that lactate is not just a by-product of extreme exercise but an important cellular energy fuel has relevance to the healing process involved in tissue injury [98,99]. Additionally, generation of NO appears to be an important player, possibly acting as a unifying molecular switch to trigger the whole cascade of the mitochondrial biogenesis process [100]. Chronic, small increases in NO levels stimulate mitochondrial biogenesis in muscle cells [101]. Moreover, it could be inferred that the generation by HWDS of increased muscle oxygenation (needed for energy production) may induce an increase in new blood vessel formation by either shear force or putatively increasing angiogenesis factors at the site of chronic muscle stimulation [102]. For example, specific adaptive responses to endurance or resistance training-like electrical muscle stimulation induce the endurance protein PGC-1G alpha. Interestingly, the level of PGC-1G alpha effects human physical performance is dependent upon an association with the PPARG gene polymorphism [103].
Understanding these newer concepts especially as they are related to loading of injured tissue could impact clinical practice. The importance of proper loading to achieve normal healing is underscored when we consider the differences observed between immobilization compared to mobilization especially with reference to scar (collagen) tissue formation in, for example, ligaments. Lo et al. [99] characterized the cellular organization of the anterior cruciate ligament (ACL) and the ovine medial collateral ligament (MCL). They compared the organization of healthy ligaments with that found in healing ligaments. Over time they found that both the ACL and MCL scars displayed discontinuities in their cellular rows. However, the scars of the MCL contained discontinuities filled with cellular projections and gap junctions, while, discontinuities in ACL scars were devoid of cells and gap junctions. The authors [104] correctly suggested that the differences between normal and scar cytoarchitecture and these discontinuities may provide the structural basis for the altered biomechanics of healing ligaments and/or represent features of an inadequate healing response. Moreover, others [105] have evaluated the changes which occurred in the epiligament, an enveloping tissue of the ligament, during the ligament healing. The results revealed that on the eighth, thirteenth and sixteenth day of post-injury, the epiligament tissue is not completely regenerated. Understanding that the tissue is not fully restored led the authors to discuss new strategies for healing including appropriate loading techniques. The use of the H-Wave® device and program is actually shown by experimentation to cause significant tissue loading in the early stages of tissue repair.
To summarize, the process of healing tissues involves many factors predominantly: 1) loading of bone, fibrous tissue and muscle; 2) NO dependent increase in blood flow; 3) increase in protein clearance at injured loci; 4) increased formation of new blood vessels or angiogenesis; 5) increased absorption of cellular lactate and 6) increased mitochondrial biogenesis (see Figure 3).
H-wave® HWDS showing proposed cellular response mechanisms derived from evidence-based medical published articles [9-26].
2. CONCLUSION
We conclude that, in addition to providing a safe, non-addicting, alternative to pain medication, HWDS may promote tissue healing. Support for this hypothesis, albeit the need for more intensive research, is derived from both animal and human studies delineated in this article [see Table 1]. Further genotyping for certain genetic antecedents for pain and tissue healing (e.g. muopioid receptor, dopamine D2 receptor, TNF-alpha, interleukins and eNOs) may significantly increase positive outcomes in the utilization of the HWDS. While, as yet, we do not have genotyping information about the reason why we are actively pursuing this novel approach.
Thus, the use of electrotherapy should enhance tissue responses that can restore structure and function after injury. Based on evidence provided by these studies, the complex interrelated series of events that commences with the release of inflammatory mediators and ends when remodeling of the repair tissue are augmented by electrotherapy. They include: cellular (NO production); humoral (release of VGEF); vascular events (increased blood flow followed by angiogenesis); increased cellular absorption of lactate; enhanced mitochondrial biogenesis, and potential induction of PPAR gamma gene with concomitant production of protein PGC-1G alpha and are a continuous sequence that reaches a homeostatic state leading to the remodeling stage.
Utilization of electrotherapy may be a wise decision by clinicians as an analgesic alternative to powerful legal opioids. With increasing knowledge and understanding of the benefit of genetic testing in terms of medication monitoring through, for example, genotyping for the metabolic P450 system and reward genes as proposed in the GARS test, analgesic therapy could be personalized
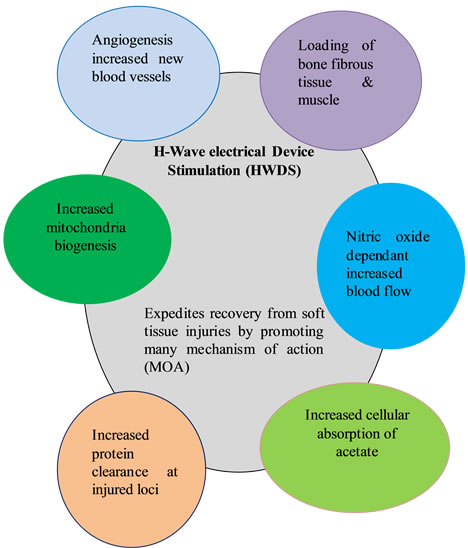
Figure 3. HWDS cellular response mechanisms.


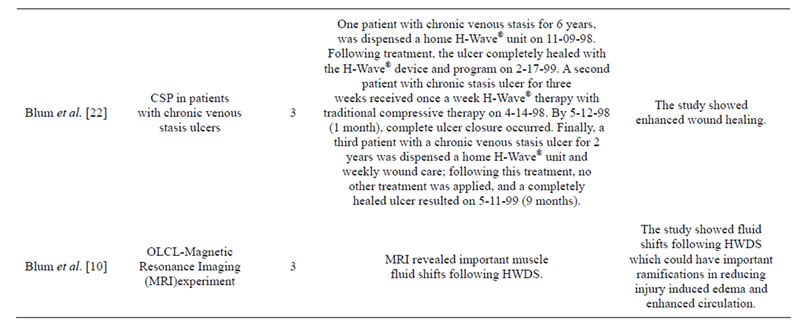
Table 1. Summary of H-Wave® pre-clinical and clinical trials.
Abbreviations: MOA, mechanism of action; RSC, randomized single-blind sham-controlled; COS, chronic observational study via post-hoc questionnaire; CSP, case study perspective; RCRS, randomized controlled rat study; RCSRS, randomized controlled sham rat study; RDBSC, randomized double-blind sham-controlled; HWDS, H-Wave® device stimulation; L-NAME, L-nitro-arginine methyl ester, SPPC Each subject served as their own control pre to post measurement, OLCT, open label clinical trial.
with reducing the risk of addiction liability [83]. Finally, these modalities may have a beneficial therapeutic impact on the dilemma of endemic of the opioid prescription in the United States and abroad [5-8,106-109].
3. ACKNOWLEDGEMENTS
The authors would like to thank the staff at Path Research Foundation NY, New York, New York, as well as financial support. The authors acknowledge the financial support of Electronic Waveform lab of Huntington Beach, California. Marlene Oscar-Berman, PhD is the recipient of grants from the National Institutes of Health, NIAAA (RO1-AA07112 and K05-AA00219) and the Medical Research Service of the US Department of Veterans Affairs. Kenneth Blum, PhD and Eric Braverman, MD are recipients of a grant from Life Extension Foundation, Ft. Lauderdale, Florida, USA.
LIST OF ABBREVIATIONS
ROM: Range of motion;
HWDS: H-Wave® device is stimulation;
NO: Nitric oxide;
MOA: Mechanisms of action;
MCL: Medial collateral ligament;
ACL: Anterior cruciate ligament;
nNOs: Endothelial nitric oxide;
DAF-FM DA: Difluorofluorescein diacetate;
TGFs-beta: Transforming growth factors-beta;
TNF-alpha: Tumor necrosis factor-alpha;
PDGF: Platelet derived growth factor;
IL-6: Interleukin-6;
NGF: Nerve growth factor;
b-FGF: Fibroblast growth factor;
IL-1 beta: Interleukin-1 beta;
VGEF: Vascular endothelial growth factor;
IF: Interferential;
Alpha 2M: Alpha 2-macroglobulin.
NOTES
Authors’ contributions: All authors equally contributed to this article.