Bright Green Luminescence from Zirconium Oxide Stabilized with Tb3+ Ions Synthesized by Solution Combustion Technique ()
1. Introduction
Since Harrison et al. [1] discovered a series of compounds with the general formula , were R2+ = Mg, Ca, Sr, Ba, Pb, Cd, Zn; R4+ = Ti, Zr, Sn; R5+ = P, As, Sb, V, which exhibited a blue broad band fluorescence, the research on host-luminescent materials is attracting considerable attention due to their excellent optical, luminescent efficiency, mechanical, electrical and thermal properties [2,3]. Among these materials, the ZrO2 or zirconia has been considered as a host material with the before characteristics. The undoped ZrO2 exists in tree isomorphic forms depending of the annealing temperature Ta; if Ta < 1170˚C the monoclinic (m) phase is present, if 1170˚C < Ta < 2370˚C ZrO2 is tetragonal (t), while above of 2370˚C ZrO2 has a cubic (c) phase [4]. However, to obtain the tetragonal and cubic room, stabilized phase is no necessary an increase of temperature since actually there are some chemical methods to stabilize this phase at room temperature, these methods include producing material with grains below a critical size; Garvie [5], Stichert [6], and Valmalette [7] studied the influence of the crystallite size on the BET superficial area, the crystallization temperature, and the phase transition temperature; doping with foreign ions such as Eu3+, Tb4+, Ca2+, Mg2+, La3+, Y4+ [2,8-16] etc.; using specific solvents; Xiulin et al. [17] synthesized pure tetragonal and monoclinic phases using polyhydric alcohols and alkil halides respectively. Stabilized ZrO2 is useful in high temperature solid oxide fuel cells (SOFC), high temperature PH sensors, and as special refractory material [12-14] phase. Moreover, it is well known that the luminescence of the zirconia can be enhanced by an doping process with rare earth such as Tb3+, Pr3+, Er3+, Eu3+, and Sm3+ ions [2,8,11-21], these today are considered the better optical activators for luminescent devices and photonics. There are various routes for the ZrO2 synthesis, among them the most used are: homogeneous precipitation [22-24], sol-gel method [13,25-27], spray pyrolysis [12], laser floating zone technique [28], solid reaction state [29], and solution combustion synthesis method [11,30-33], this last method is quite simple, fast and economical in which an oxidizer and a fuel in an highly exothermic redox chemical reaction stoichiometric produce ZrO2, H2O vapor, N2, and CO2. In this work, using Zirconil Nitrate ZrO(NO3)3 as oxidizer, urea (H2NCONH2) as fuel and terbium chloride as dopant, undoped and terbium doped ZrO2 is synthesized by solution combustion technique as a function of Tb3+ ion concentration in W%, later annealed at 900˚C during 20 hours. Under ultraviolet (325 nm) radiation excitation their luminescent properties are investigated, as a function of the doping concentration.
, were R2+ = Mg, Ca, Sr, Ba, Pb, Cd, Zn; R4+ = Ti, Zr, Sn; R5+ = P, As, Sb, V, which exhibited a blue broad band fluorescence, the research on host-luminescent materials is attracting considerable attention due to their excellent optical, luminescent efficiency, mechanical, electrical and thermal properties [2,3]. Among these materials, the ZrO2 or zirconia has been considered as a host material with the before characteristics. The undoped ZrO2 exists in tree isomorphic forms depending of the annealing temperature Ta; if Ta < 1170˚C the monoclinic (m) phase is present, if 1170˚C < Ta < 2370˚C ZrO2 is tetragonal (t), while above of 2370˚C ZrO2 has a cubic (c) phase [4]. However, to obtain the tetragonal and cubic room, stabilized phase is no necessary an increase of temperature since actually there are some chemical methods to stabilize this phase at room temperature, these methods include producing material with grains below a critical size; Garvie [5], Stichert [6], and Valmalette [7] studied the influence of the crystallite size on the BET superficial area, the crystallization temperature, and the phase transition temperature; doping with foreign ions such as Eu3+, Tb4+, Ca2+, Mg2+, La3+, Y4+ [2,8-16] etc.; using specific solvents; Xiulin et al. [17] synthesized pure tetragonal and monoclinic phases using polyhydric alcohols and alkil halides respectively. Stabilized ZrO2 is useful in high temperature solid oxide fuel cells (SOFC), high temperature PH sensors, and as special refractory material [12-14] phase. Moreover, it is well known that the luminescence of the zirconia can be enhanced by an doping process with rare earth such as Tb3+, Pr3+, Er3+, Eu3+, and Sm3+ ions [2,8,11-21], these today are considered the better optical activators for luminescent devices and photonics. There are various routes for the ZrO2 synthesis, among them the most used are: homogeneous precipitation [22-24], sol-gel method [13,25-27], spray pyrolysis [12], laser floating zone technique [28], solid reaction state [29], and solution combustion synthesis method [11,30-33], this last method is quite simple, fast and economical in which an oxidizer and a fuel in an highly exothermic redox chemical reaction stoichiometric produce ZrO2, H2O vapor, N2, and CO2. In this work, using Zirconil Nitrate ZrO(NO3)3 as oxidizer, urea (H2NCONH2) as fuel and terbium chloride as dopant, undoped and terbium doped ZrO2 is synthesized by solution combustion technique as a function of Tb3+ ion concentration in W%, later annealed at 900˚C during 20 hours. Under ultraviolet (325 nm) radiation excitation their luminescent properties are investigated, as a function of the doping concentration.
2. Experimental Details
ZrO2 doped with Tb3+ ions is obtained by an solution combustion redox chemical reaction stoichiometric using as raw materials ZrO(NO3)2 (oxidizer), H2NCONH2 (fuel) and TbCl3 as doping source under the follow theoretical reaction chemical:
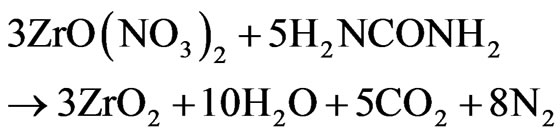 (1)
(1)
The Equation (1) was obtained by taken into account the oxidizer/fuel molar radio (O/F = 1) required for a stoichiometric mixture which is determined by summing the total oxidizing and reducing valencies in the oxidizer compound and dividing it by the sum of the total oxidation and reducing valencies in the fuel compound [30]. Accordingly for the complete combustion of zirconyl nitrate-urea mixture, the molar ratio becomes 10/6 = 5/3, the molar balanced Equation (1) was obtained with this value. Using the atomic weight concept, the Equation (1) was translated to grams/mol and used to obtain 5 gr. of ZrO2 for all the Tb3+ ion concentrations. The Tb3+ ion concentration was in the range from 0 to 10 (0, 1, 2, 3, 5 and 10) weight percent (W%) in relation to the ZrO2 content. From the grams/mol equation stoichiometric (1), 9.78 gr of ZrO(NO3)2 was mixed with 4.057 gr of H2NCONH2 plus the grams number corresponding to each Tb3+ ion concentration and 20 ml of water on a flask glass and put on a hot plat at 450˚C, after a few minutes the mixture boils, foams, ignites, and burns with an incandescent flame at a temperature of 1500˚C [30] producing 5 gr of pure ZrO2 plus water vapor, CO2, and N2. All produced powders were annealed at 900˚C during 20 hours.
The material produced was characterized by X-ray diffraction (XRD) using a Philips PW 1800 diffractometer with Cu Kα radiation (1.5406 Å), the morphology of the ZrO2 powders obtained was studied using an scanning electron microscopy (SEM) JEOL JSM 840 A, and the photoluminescence (PL) spectra were recorded using a spectrofluorimeter Fluoro Max-P that uses a Xenon lamp as excitation source.
3. Results and Discussion
Figure 1 shows the XRD patterns for the ZrO2 polycrystalline powder as a function of the Tb3+ doping concentration. It is observed that the XRD pattern for undoped

Figure 1. X-ray patterns of ZrO2/Tb3+ annealed at 900˚C by 20 hours showing the evolution of the crystalline phase as a function of the Tb3+ ions concentration.
zirconia sample shows various peaks that are associated to two crystalline phases: monoclinic (m), and tetragonal (t) phases. The peaks centered at 24.56˚ (1,1,0)m, 28.40˚ (−1,1,1)m and 31.41˚ (1,1,1)m are characteristic peaks of the monoclinic phase, similarly, the peaks centered at 30.34˚ (1,0,1)t, 35.40˚ (0,1,1)t, 50.51˚ (2,1,1)t and 60.40˚ (1,1,2)t indicates the presence of the tetragonal phase. The aggregation of TbCl3 for all concentrations in the redox reaction between fuel-oxidizer to doping ZrO2 with the Tb3+ ion results in an phases composition and size crystallite change; the proportion of the phases monoclinic and tetragonal was obtained as an function of the Tb3+ ion concentration using the not entirely justified theoretically Garvie’s equation [34]:
 (2)
(2)
where I is the integrated intensity in each peak and m and t indicate for monoclinic and tetragonal phases. The average crystallite sizes were calculated by means of the Scherrer formula
D = (0.9λ)/βcos(θ)(3)
where λ is the X-ray wavelength, β is the half-width of the strongest diffraction peak and θ is the diffraction angle. The graphics of the Equations (2) and (3) are shows in Figures 2(a) and (b) respectively. From Figure 2(a) can be see that increasing the Tb3+ ion concentration the content of the metastable t phase was increased reaching the maximum for 5 W% of the ion dopant. For the content of the monoclinic phase this decrease with the increase of the ion concentration. The evolution of the av-
 (a)
(a) (b)
(b)
Figure 2. Evolution of phase composition (a), and size crystallite (b) as function of Tb3+ ion concentration for samples annealed at 900˚C by 20 h. Lines are drawn as guides for the eyes.
erage crystallite size as an function of the ion concentration is shown in Figure 2(b), it is observed that the size average for the t phase increase, while the m structure diminishes with the concentration. Also, the XRD pattern do not show a phase attributed to the presence of Tb2O3, suggesting that the Tb3+ ions are well inserted in the zirconia host. The tetragonal phase is consolidated as an effect of the increasing of the presence of the Tb3+ ions in the ZrO2 crystalline structure indicating that it is possible to stabilizing the room temperature tetragonal phase of zirconia by doping it with rare earth ions. Also, a sliding of the peaks to lower 2Ø values respect to the undoped zirconia showing small changes in the lattice parameters is observed due to the incorporation of the terbium ions without changes in the full-width at half maximum (HWFM) of the peaks, indicating a grown uniform of the zirconia crystallites.
R. C. Garvie [35] was the first in to propose that the metastable tetragonal and cubic phases can be stabilized at room temperature when the crystallite size is bellow a critical size (which Garvie fixed in 300 Å) primarily due to very high surface energy associate with it, when the crystallite size exceeds this critical size by means of heat, the transformation of t and c metastable phases to monoclinic one occur due to decrease in surface energy; Filipovich and Kanilina [36] showed that the stable high temperature polymorph of a crystal can be stabilized at temperatures below its normal transformation temperature at some critical size if the high temperature polymorphic has a reduced surface free energy with respect to low temperature structure. Moreover, it has also been the cause of first-principles studies of phase transitions and doped-induced phase stabilization, for example the work by Hansen [37] on free-energy calculations, Kwok [38] and French [39] on optical properties and electronicstructure calculations, band an cluster-based electronicstructure calculations [36-38]. These results are compatibles with the results obtained by F. Ramos-Brito et al. for Pr3+ ion doped zirconia [11] who found that the monoclinic phase transforms to tetragonal phase when Pr3+ ion concentration exceeds 5% and with the results of W. Cordova-Martinez et al. [9] that experimentally showed that it is possible to obtain up to 73% of tetragonal phase by doping the ZrO2 with 2 mol% of Sm2O3 or Tb2O3 and annealing at 1000˚C. More recent theoretical and experimental studies carried out by French et al. [39] revealed that the high density of the doped-induced oxygen vacancies in the ZrO2 electronic structure play a important role in the t-ZrO2 stabilization; these oxygen vacancies are present in stabilized materials at high concentrations, for example 9.5 mol% Y2O3 stabilized c-ZrO2 contains 4.75% oxygen vacancies. These oxygen vacancies are localized adjacent to Zr atoms reducing the symmetry and coordination of the Zr site.
Figures 3(a) and (b) show the photoluminescence spectrum for the sample of undoped ZrO2 annealed at 900˚C by 20 hours and for the sample of ZrO2 unannealed respectively, the samples were UV radiated with a wavelength of 325 nm. The emission spectrum are alike for both samples and present a single and very broad band covering the range 400 - 600 nm peaking at 480 nm, but the intensity band of the annealed sample is higher than that for unannealed sample. From the XRD results (see Figure 1) it is concluded that the undoped ZrO2 present two different phases: monoclinic and tetragonal, this result could indicate that both phases contribute to the PL emission bands. This result is in agreement with the reported by Numan Salah et al. [40]. From the results
 (a) (b)
(a) (b)
Figure 3. Photoluminescence spectra of undoped ZrO2 annealed at 900˚C during 20 hours (a) and unannealed (b).
reported in the literature it is known that the PL emission band of ZrO2 can be attributed to the formation of energy levels or electron/tramps states created on the ZrO2 surface in which electron transitions from the surface tramp states in the conduction band to lower energy levels near to the valence band can occur [34]; Harrison et al. [1] in their luminescence study of ZrO2 and ternary zirconate compounds showed that the zirconium ion plus its surrounding oxygen ion constitute the most conspicuous component of the luminescent center; their emission spectra for ZrO2 also shows, as in our case, a broad band centered at 480 nm under similar excitation wavelength. Harrison attributed this broad band to the existence of the zirconium-oxygen ion complex. With respect to the monoclinic phase present in the ZrO2 powders, McCullough et al. [41] studying the crystalline structure of monoclinic ZrO2 found that the Zr4+ ion is surrounded by 7 oxygen ions at distances (Zr-O) ranging from 2.04 to 2.26 Å, thus the emission in ZrO2 can be attributed to a distortion in the symmetry of the surrounding oxygen ions as showed by Harrison. Tahir et al. [42] found that exciting ZrO2 with 270 nm radiation, a broad emission band ranging between 300 - 500 nm, peaking at 376 nm was observed; they have attributed this broad emission band to the narrow particle size distribution caused by the nanometric character of the ZrO2 particles size, producing an inhomogeneous broad distribution of surface or defect states. Add to the before, the defects formed by the interfacing sites between the phases monoclinic and tetragonal, configure an wide band gap of the ZrO2 that contain new energy levels and electron tramps that can producing a lot of emissions.
Figure 4 shows the room temperature emission spectra of ZrO2/Tb3+ powder annealed at 900˚C during 20 hours as a function of Tb3+ ions concentration; the powders

Figure 4. Photoluminescence emission spectra of ZrO2/Tb3+ powders, as a function of Tb3+ ions concentration.
were excited by ultraviolet radiation (325 nm). All the spectra for doped samples show four principals emission bands peaking at about 490, 545, 588 and 620 nm; these peaks, correspond to the electronic transitions that occur in the Tb3+ ion, namely the 5D4→7F6, 5d4→7F5, 5D4→7D4, and 5d4→7F3 transitions. The strongest emission peak at 545 nm is responsible for the characteristic Tb3+ ion green light emission. These results are compatibles with those previously obtained by Garcia-Hipolito et al. [15] for ZrO2:Tb3+ thin films by pneumatic spray pyrolysis and by B. Marí et al. [11] for the same product in powders synthesized by solution combustion. Figure 5 shows the relative emission intensity, as a function of the Tb3+ ion concentration, for ZrO2/Tb3+ powders considering only the emission intensity of the band centered at 545 nm; here it is observed a maximum intensity for 2 wt% doping concentration. After this value, a decrease of PL emission intensity is exhibited due to excess of activators ions, effect known as concentration quenching. In the literature, it is suggested that, at high doping concentration, the excitation energy can migrate from one luminescent center to another and eventually reach a sink (lattice defect), from which non-radiative processes can dissipate this excitation energy. This concentration quenching will not appear at low doping concentrations because the average distance between activators ions is so large
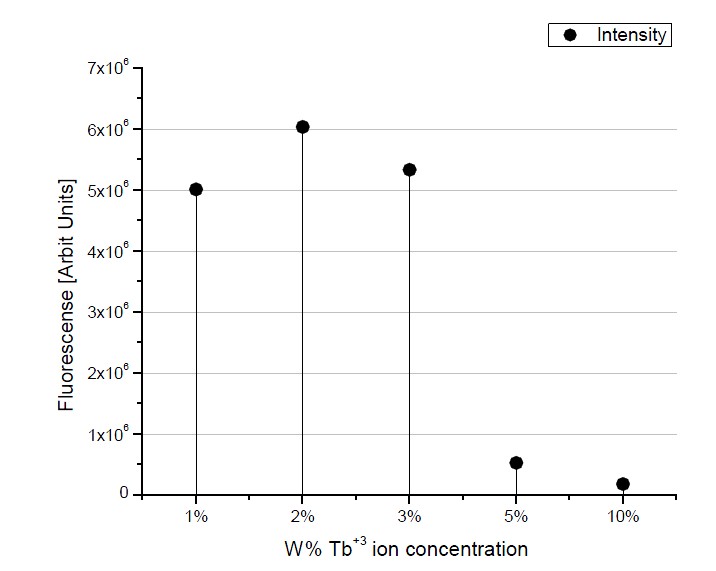
Figure 5. Relative intensity of the emission peak centered at 545 nm of ZrO2/Tb3+ powders as a function of the Tb3+ ions concentration.
that the migration is prevented and the sinks are not reached [32].
Figure 6 shows the surface morphology and size of the ZrO2/Tb3+ particles doped with 2 W% of Tb3+ ions, obtained by SEM. It is observed that the morphology and size of the particles is more or less uniform and similar to ovoid-like morphology. The ovoid-like particle size is less than 1.0 µm. The SEM images also exhibits pores and voids in the ZrO2/Tb3+ sample, it is because in solution combustion synthesis the oxidizer-fuel redox reaction is performed in short times and evolve emitting a lot of gases like N2, CO2 and H2O that can yields particles with pore and voids.
4. Conclusions
In this contribution, results about powders of ZrO2 doped with 0, 1, 2, 3, 5, and 10 wt% of Tb3+ ions, are reported. These powders were synthesized by a simple and economical solution combustion technique, using zirconyl nitrate hydrate as oxidizer, urea as fuel and terbium chloride as doping impurity. The XRD studies revealed that the undoped powders (ZrO2) show the room temperature monoclinic stabilized phase and the size-effect stabilized room temperature stabilized tetragonal phase.
Studies of the phases composition revealed that when the Tb3+ increase, the content of the monoclinic phase decreases while the content of the tetragonal phase increases.
Undoped tetragonal and monoclinic phases exhibited intense green photoluminescence by means of a broad band peaking at 480 nm. Presumably that PL emission is due to structural defects of the host lattice.
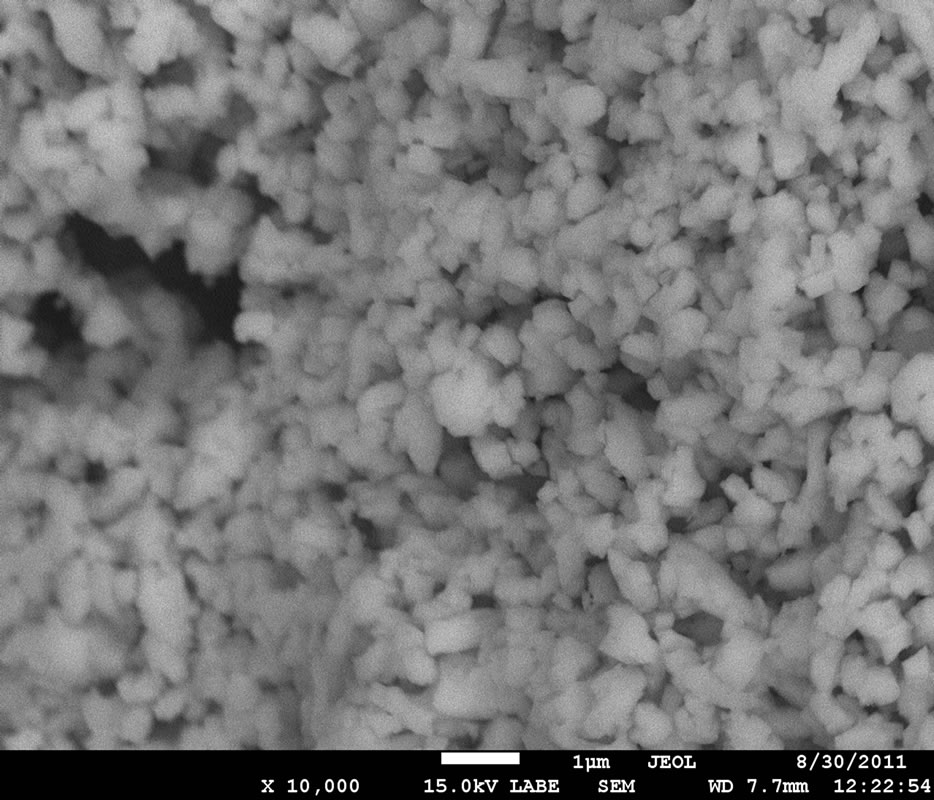
Figure 6. SEM micrograph of ZrO2/Tb3+ powders annealed at 900˚C during 20 hours, in this case the doping concentration was 2 wt%.
All ZrO2/Tb3+ samples showed four emission bands peaking at 490, 545, 598, and 620 nm corresponding to the 5D4→7Fn, n = 3 - 6, electronic transitions of the Tb3+ ion, when excited by ultraviolet radiation (325 nm). The sample doped with a 2 wt% of the Tb3+ ions showed the maximum green PL emission intensity. The observed green emission is very strong and it is possible to perceive it in normal room light with the naked eye.
SEM micrographs of the ZrO2/Tb3+ powders showed a morphology constituted by particles with indefinite form similar to ovoids approximately of a micron in size. The estimated crystallite size, calculated by means of the Scherrer formula, was about 30 nm.
5. Acknowledgements
The authors wish to thank to C. Falcony (CINVESTAV-IPN) for his support in carrying out the PL measurements, Adriana Tejeda for her support in carrying out the XRD studies and Omar Novelo for his support in carrying out the SEM studies and M. A. Canseco Martínez by his support in chemistry reactions.