1. Introduction
Digenean parasites of Apocreadiidae family from fish of the Western Mediterranean have received a considerable attention in recent year [1]. Pagellus erythrinus, in Mediterranée westerner and in particular in Algeria few works exists on the parasitofaune of this marine host, this deficiency aroused our interets in investigated by advantage. This Teleostei fish takes a preponderant places as link in the throphodynamic chain and may be used as an intermediary or definitive host into the cycle of many Digenea parasite. They are caught in the Oran bay, Algeria (Figure 1), and dissected, for helminth investigation. These fishes are parasitized largely by a small new species of Digenean, dwelling in intestine of their hosts. The species is described in the present work.
2. Materials and Methods
The Digeneans recovered from intestine of P. erythrinus are set into alcohol of 70 volumes, stained some minutes in boracic Carmin of Grenacher, dedifferentiated in
chlorhydric acid, dehydrated in successive baths of alcohol of 70, 85, 95 volumes, then in two baths of absolute ethanol of 100 volumes. They are cleared in Eugenol and mounted in Canada balsam. They are drawn with the camera lucida of an Olympus microscope.
3. Results
Fifty P. erythrinus are dissected between October 2010 and June 2011. Seasonal variations in the presence of Digeneans and variations linked with the size of the hosts are noted. For the recovered new species, the higher period occurs in spring, between April and June 2011, in 75% of fishes measuring 13 to 19 centimetres, and in 30% to 45% of fishes measuring 7 to 12 centimetres.
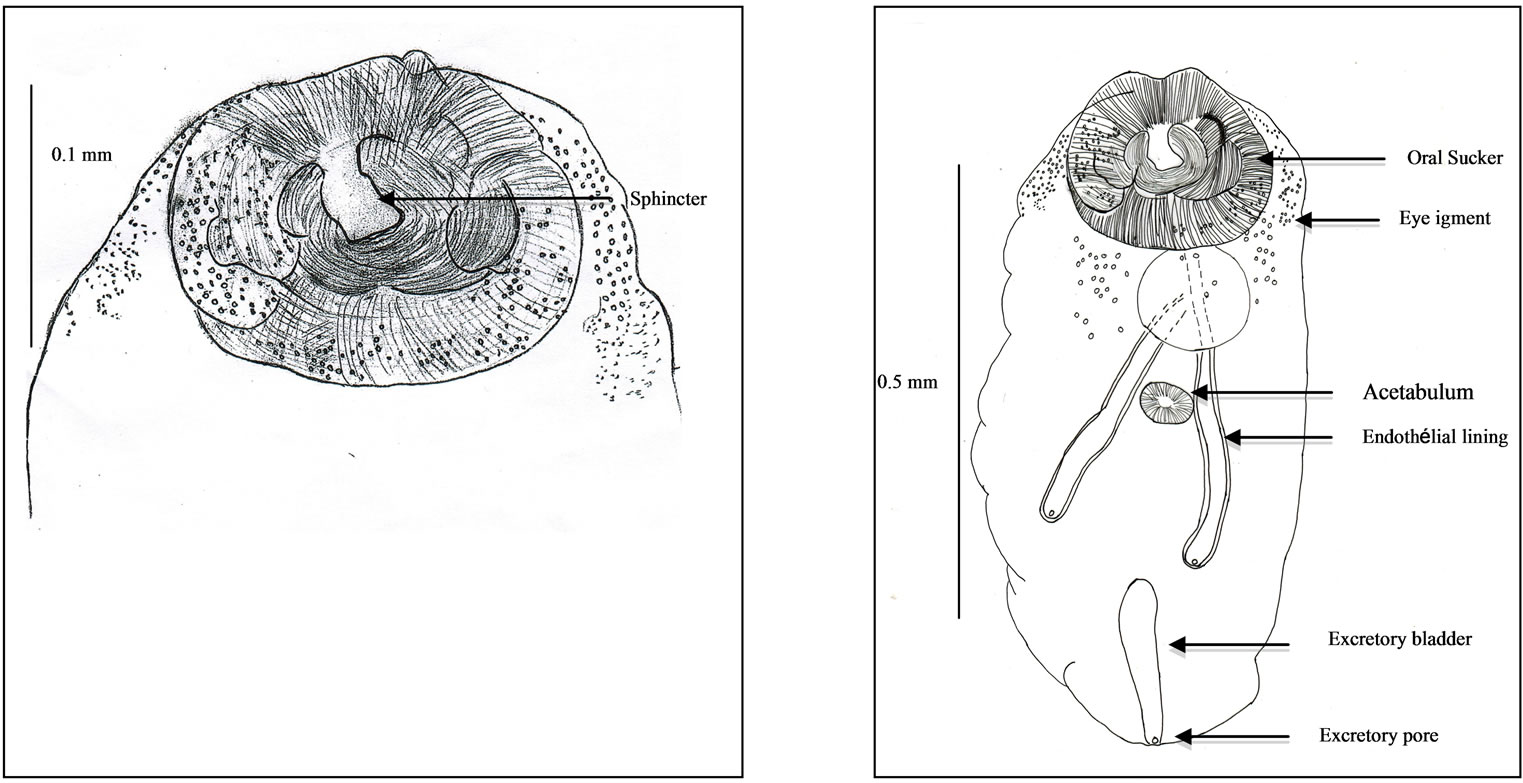
A convenient period is observed in autumn between October and December 2010 and during the last part of winter, in February-March 2011 in 30% of fishes. No parasite in January 2011. No dissection in July and August Description of our new species (Figures 2-4). It is based on observations of seven specimens, showing various stages of development, from which one immature worm. One immature worm devoid of genital apparatus, displays a very large oral sucker, a small acetabulum, a digestive apparatus, and an excretory apparatus, only. Juvenile worms with moreover, a genital apparatus devoid of egg. Ovigerous worms (Table 1), with all the apparatus and eggs. Ovigerous worms. Details, lobes in oral sucker, level of ovary, Melhis gland, Laurer’s canal, excretory anterior arms.
Main distinctive features. Very large lobed oral ucker, provided with an inner powerful muscular sphincter, with an outer circular and longitudinal musculature. Mouth opening in an irregular hexagonal aperture flowed by a narrow slit. Small acetabulum weakly muscular. Three genitalia in tandem, in the hindbody: an ovary transversally elongated, followed by two testes lobed heartshaped. Extracaecal vitelline follicles, converging in the hindbody.
Sinuous pear-shaped seminal vesicle, near the cetabulum, slightly globular short prostate, surrounded by few prostatic cells, narrow ejaculatory duct. No cirrus pouch.
Creased distal uterus. Spherical genital atrium. Caeca with subterminal ani. Short excretory bladder I-shaped (Figure 2(b)).
Body. About twice longer than broad. The average length × width, measured on seven specimens, is 0.62 × 0.34 millimetre. Body coat smooth, without spine. Antero-dorso-lateral eye pigment, under the oral sucker. Undulating body side edges.
Suckers. Oral sucker very large (Figure 2(a)), with a diameter of about 0.18 millimetre. It includes, in the in-
 (a) (b)
(a) (b)
Figure 2. Immature worm. (a) Oral sucker and sphincter; (b) General view.
 (a) (b) (c)
(a) (b) (c)
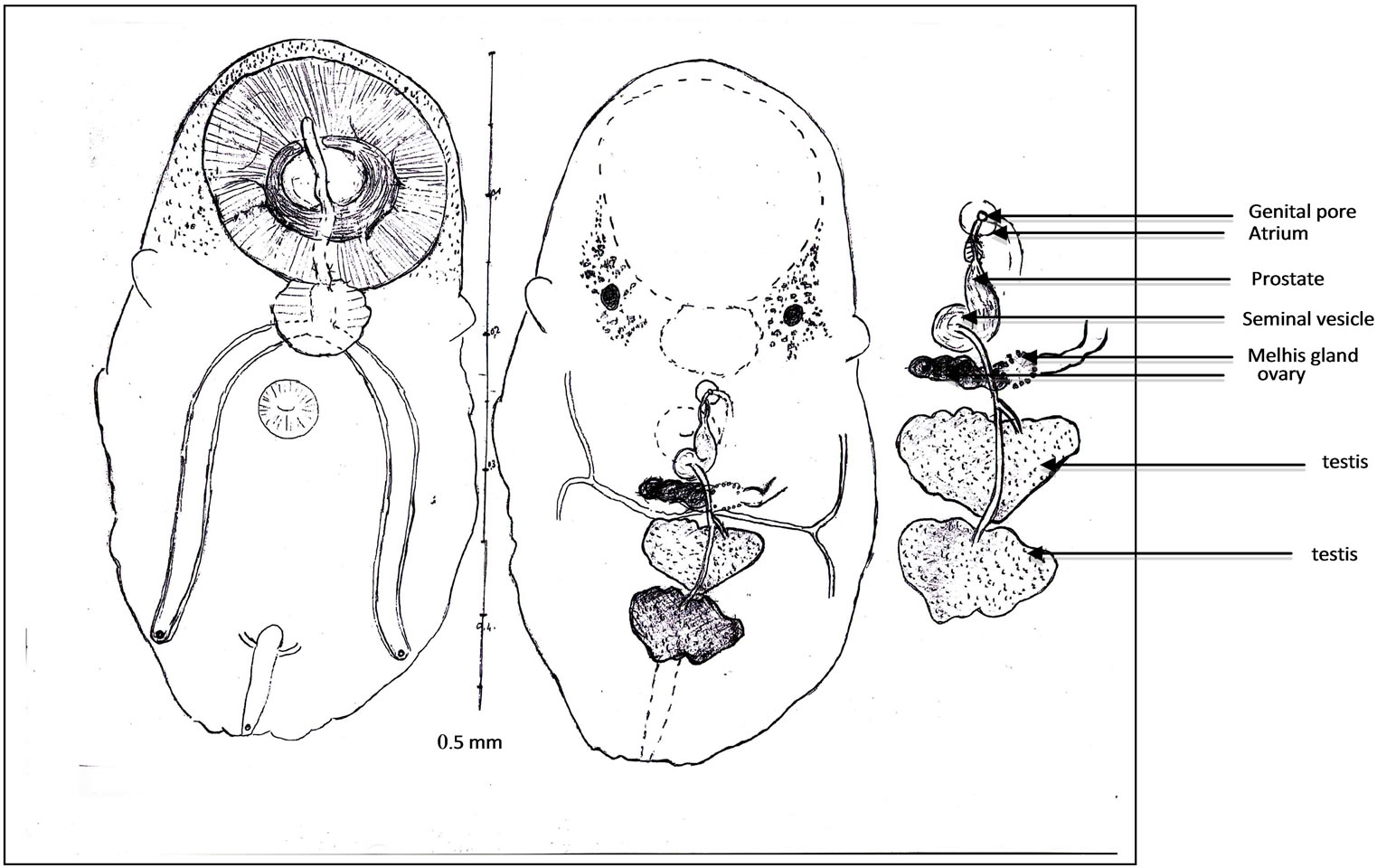
Figure 3. Juvenile worm (a); General view. Oral sucker and sphincter. Acetabulum. Digestive apparatus, buccal slit, pharynx, caeca, endothelial lining, ani. Eye pigment. Ovary, Melhis gland, vitelloducts. Testes, spermiducts. Excretory bladder, ending of excretory arms. Excretory pore, Scale (b); Terminal genitalia. Seminal vesicle, prostate, prostatic cells. Ejaculatory duct. Proximal uterus. Distal uterus. Atrium. Genital pore, Scale (c).
 (a)
(a)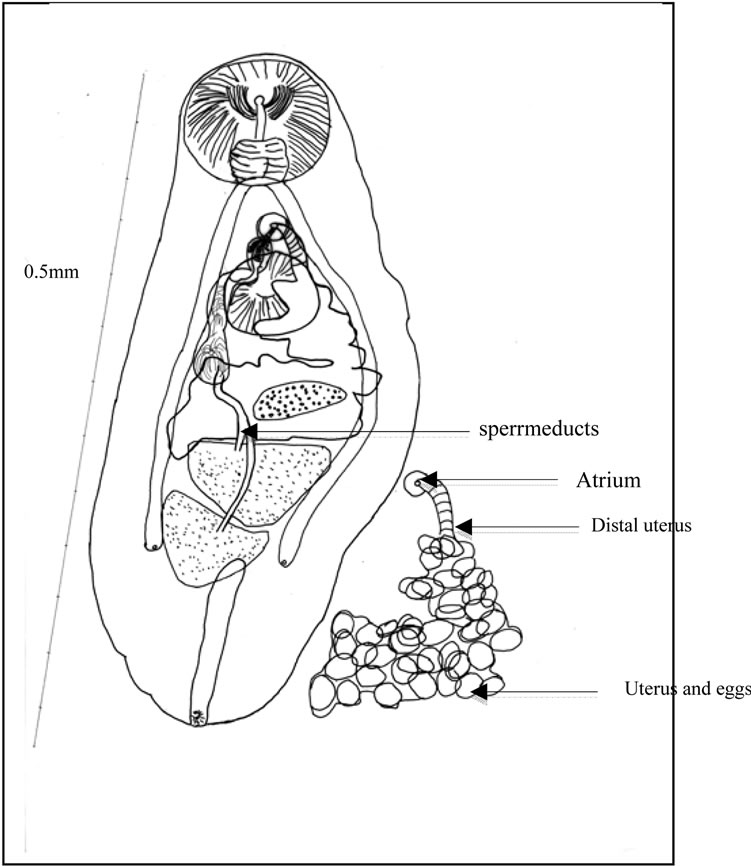 (b)
(b)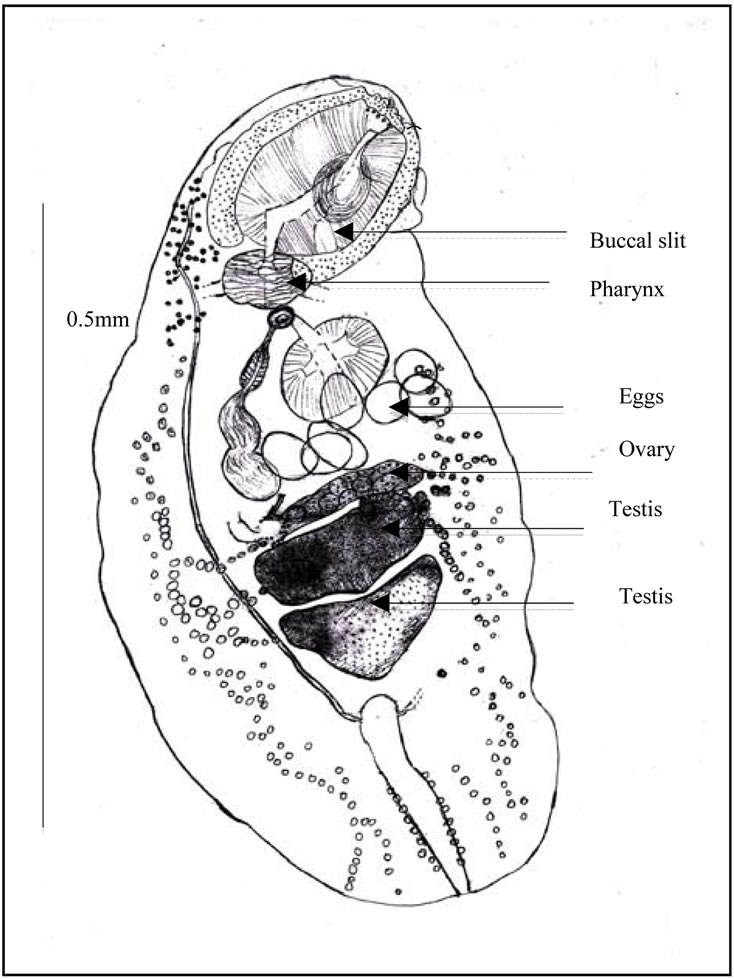 (c)
(c)
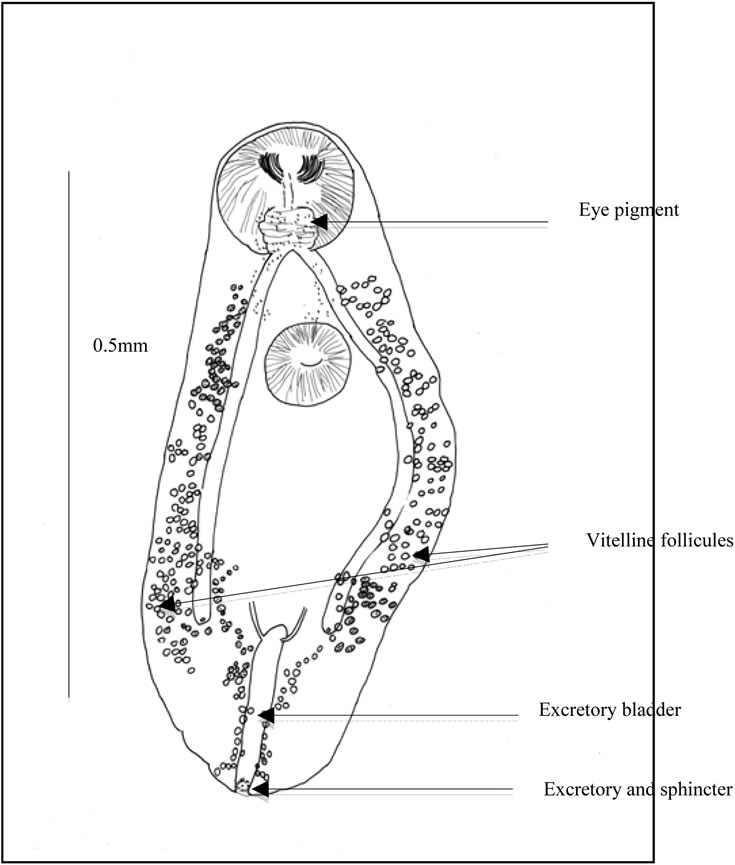
Figure 4. Ovigerous worm (a) General view. Caeca. Uterine outline. spermiducts. Seminal vesicle. Prostate. prostatic cells. Ejaculatory duct. Distal uterus. Atrium. Genital pore. Uterus and eggs; (b) Scattered eye pigment. Vitelline follicles. Excretory bladder. ending of excretory arms. Excretory pore and sphincter; (c) Buccal slit. Pharynx. Testis. Eggs. Ovary.
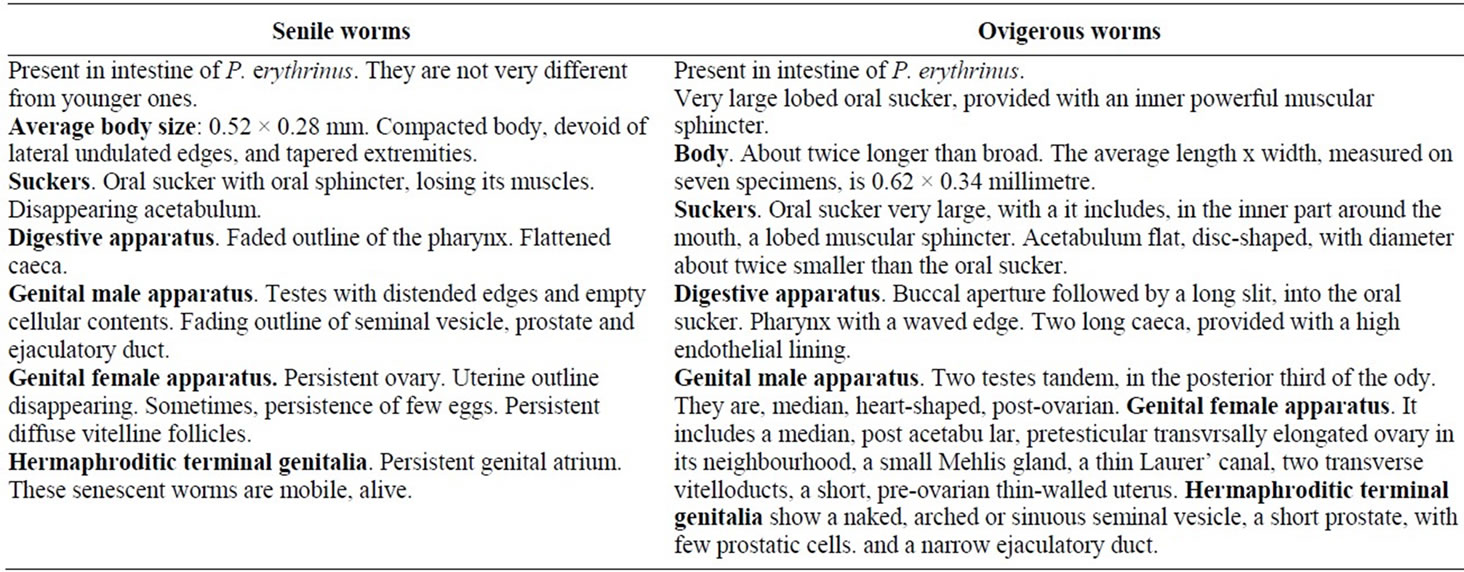
Table 1. Comparaison of Sénile and ovigerous worms in Pagellus erythrinus host.
ner part around the mouth, a lobed muscular sphincter. And in the outer part, circular and longitudinal muscles, and numerous gland like cells.
Acetabulum flat, disc-shaped, with diameter about twice smaller than the oral sucker.
Ocular pigment. In immature worms, two dark eyes are conspicuous dorsally, surrounded by broad grains. In ovigerous worms, the ocular pigment is scattered, pulverized in minute grains (Figure 2(b)).
Digestive apparatus. Buccal aperture followed by a long slit, into the oral sucker. Pharynx with a waved edge. Two long caeca, provided with a high endothelial lining (Figure 3(b)), on their inner wall. Each one opens by a subterminal anus.
Genital male apparatus. Two testes tandem (Figure 3(c)), in the posterior third of the body. They are, median, heart-shaped, post-ovarian. The first testis measures, in its greatest dimension, 0.10 mm (>0.07, <0.12). The second one measures, in its largest dimension, 0.15 mm (>0.08, <0.24). They can overlap slightly in old specimens.
The terminal genitalia show a naked, arched or sinuous seminal vesicle, a short prostate, with few prostatic cells and a narrow ejaculatory duct. Seminal vesicle bypasses part of the acetabulum. Ejaculatory duct ends in genital atrium. No cirrus pouch.
Genital female apparatus. It includes a median, post acetabular, pretesticular transversally elongated ovary. It measures, in its greatest dimension, 0.10 mm (>0.06, <0.14). In its neighbourhood, a small Mehlis gland, a thin Laurer’ canal, two transverse vitelloducts, a short, pre-ovarian thin-walled uterus. In juvenile specimens, uterus is without egg. In older specimens, it contains a relatively small number of eggs (2 or 4, or 6, or 10, and only once, 40 eggs). These eggs are light yellow coloured, thin-shelled, large. Average size, 0.030 × 0.024 mm. The distal part of the uterus transversally creased, ends in genital atrium, just before the median genital pore, near the intestinal bifurcation. Vitelline follicles are spread in two narrow bands, along the outer edge of the caeca. They converge in posterior area of the body.
Excretory apparatus. The urinary bladder is short, I-shaped. It reaches the lower level of the second testis. Its anterior part receives two thin excretory ducts coming from the forebody. The subterminal excretory pore is closed by a small sphincter.
Senile worms (Table 1). Besides the typical stages, senile worms are present, in intestine of P. erythrinus. hey are not very different from younger ones. But they appear altered. They are described briefly, underneath, without drawing.
Average body size: 0.52 × 0.28 mm. Compacted body, devoid of lateral undulated edges, and tapered extremities.
Suckers. Oral sucker with oral sphincter, losing its muscles. Disappearing acetabulum.
Digestive apparatus. Faded outline of the pharynx. Flattened caeca.
Genital male apparatus. Testes with distended edges and empty cellular contents. Fading outline of seminal vesicle, prostate and ejaculatory duct.
Genital female apparatus. Persistent ovary. Uterine outline disappearing. Sometimes, persistence of few eggs. Persistent diffuse vitelline follicles.
Hermaphroditic terminal genitalia. Persistent genital atrium.
These senescent worms are mobile, alive.
Identification of our species to a family and subfamily. Our batches of juvenile and ovigerous worms display several characteristics observed in Apocreadiidae Skrjabin 1942, Schistorchiinae [2] and Postporinae, [3], which khave been reviewed by Cribb in Jones et al. (2005) [1].
As Schistorchiinae, they have an oral sphincter in a large oral sucker. A small acetabulum near the intestinal bifurcation. Eye pigment in antero-dorsal region. Terminal male genitalia devoid of cirrus pouch, showing; a sinuous seminal vesicle, a prostate, and a thin ejaculatory duct. Genitalia including ovary and testes tandem.
Yet, in our species, the gonads are in the hindbody.
Schistorchiinae includes five genera, from which, Sphincteristomum [4] (Figure 4(c)), and Sphincterostoma [5] (Figure 4(b)), with two testes in forebody and Schistorchis Lühe, 1906, Megacreadium Nagaty, 1956 Neomegacreadium [6], with nine to thirteen testes, in one or two files.
As Postporinae, juvenile and ovigerous worms from our species, have an ovary and two testes tandem in the hindbody.
The Postporinae subfamily includes one genus with two species Postporus epinepheli [7,8] and P. mycteropercae [7,8] (Figure 4(a)). This genus shows, anterior eye pigment, an ovary and two testes tandem in hindbody, lateral extra caecal vitellus as our species. It differs from our species by its oral sucker devoid of sphincter, its blind caeca, its genital pore posterior to acetabulum, its very long excretory bladder reaching the pharyngeal level, in the first species and the intestinal bifurcation, in the second species Among the Apocreadiidae, our new species appears closely related with genera Sphincteristomum, Sphincterostoma, Schistorchiinae, and Postporus, Postporinae (Figure 4).
Comparison of our species with Sphincteristomum. The genus admits two species:
The type-species S. acollum [4], described in Balistidae fish Abalistes stellaris off the north coast of Vietnam and S. aniferum [9] (syn. Lobatotrema aniferum) from a Balistidae off Fiji island between New Caledonia and New Zealand.
Similarities. Like S. acollum, our species has an obvious sphincter in the oral sucker, a transversally elongated ovary, two lobed heart-shaped testes, a bilateral ondulating edge of the body and two terminal caeca open in ani.
Like S. aniferum, our species has a peculiar large oral sucker with minute longitudinal and transverse muscles, caeca with an inner endothelial lining and opening in ani.
Differences. S. acollum and S. aniferum have gonads in anterior or middle region of the body. Our species has gonads in posterior region.
S. acollum and S. aniferum have intracaecal and extracaecal vitelline follicles. Our species has, mainly, two narrow extra caecal bands of vitelline follicles.
Comparison with Sphincterostoma (Table 2). The genus admits one species:
The type-species S. branchiostegi [5], described in Branchiostegus japonicus off the west coast of Japan. The second one is Sphincterostoma sp [9] from a Balistidae, probable Balistapus, off Fiji island.
Similarities. Like S. branchiostegi, our species is present in intestine of its host, at different stages of development, according to Yamaguti, 1937 [5].
Differences. The two species of Sphincterostoma have a lateral and ovoid ovary. Our species has a median transversally elongated ovary. In S. branchiostegi, the oral sphincter surmounts the oral sucker. The I-shaped excretory bladder is long and reaches the anterior edge of the first testis. Location of Sphincterostoma sp. Manter, ingills of the host.
No mention or drawing of endothelial lining in caeca of the two Sphincterostoma. Our species has an endothelial lining in caeca, at least in juvenile and ovigerous specimens Comparison with Postporus (Table 2). The genus admits two species P. epinepheli [8], type-species, and P. mycteropercae [8], described respectively in Epinephelus morio and Mycteroperca venenosa, Serranidae off the Atlantic American coast of Florida.
Similarities. Eye pigment. Gonads in the hindbody. Vitelline follicles mainly extracaecal.
Differences. Spiny body. Absence of oral sphincter. Ovoid ovary. Post acetabular terminal male genitalia. Very long I-shaped excretory bladder.
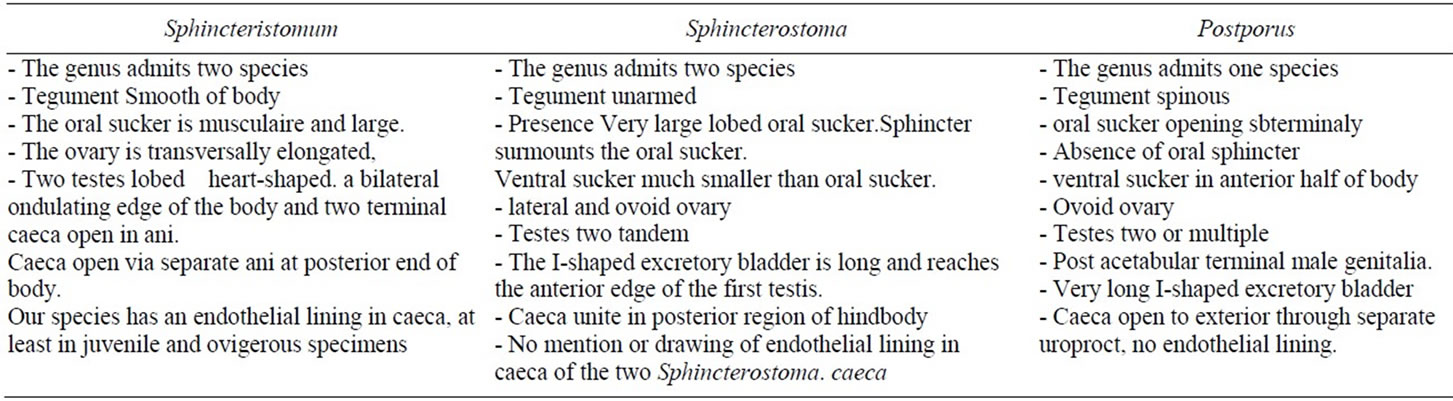
Table 2. The differences morphological of genus in Apocreadiidae family.
Generic identification of our species. They appear very closely related of genus Sphincteristomum. And they display characteristics of a new species.
Specific identification of Sphincteristomum mediterraneae n.sp.
Specific name, S. mediterraneae, because of its presence in the Mediterranean Sea.
Type locality. Oran bay, Algeria.
Host-type. Pagellus erythrinus, Sparidae.
Micro-habitat: intestine.
Holotype and paratype: samples n˚ 130 TO2, 135 TO3.
Other samples n˚ 127 TO1, 135 TO 4.
Name: Sphincteristomum mediterraneae Abid-Kachour, Mouffok and Boutiba.
4. Discussion
Geographic locations of Schistorchiinae and their final hosts.
Schistorchiinae subfamily sensu Cribb (2005) [1] contains few genera, described in several families of marine fishes spread all around the world. From East to West: Sea of Japan opening in North Pacific Ocean, Tonkin Bay on Sea of China, South Pacific Australian Ocean, Indian Asian Ocean, Indian African Ocean, Red Sea, Mediterranean (present result), and Atlantic American Ocean.
The genera of final hosts are enounced, as in authorsdescriptors. When it is possible, the family of fish is mentioned according to Tortonese (1973) [10].
Schistorchis carneus Lühe, 1906 from Tetrodon stellatus Tetraodontidae, near Sri Lanka, Indian Ocean. S. haridis [11] from Pseudoscarus harid, Red Sea.
S. seychellesiensis Toman,1989, from Cantherines pardalis (Rupp, 1837), Indian Ocean. S. paruchini Kurochkin, 1974 from Navodon australis, South Pacific Ocean.
Megacreadium tetrodontis [12] from Tetrodon, in Red Sea.
Neomegacreadium okinawanum [6], from Triodontidae of Japanese and adjacent waters.
Sphincterostoma branchiostegi [5] from Branchiostegus japonicus in Sea of Japan. S. sp Manter, 1963 from a probable Balistapus Balistidae off Fiji.
Sphincteristomum acollum from Abalistes stellaris Balistidae in Tonkin Bay.
S. aniferum from triggerfish Balistidae from Fiji, in South Pacific Ocean.
S. mediterraneae n. sp. in Pagellus erythrinus Sparidae Although the helminthofauna of Mediterranean is well known, since a long time, and although the Schistorchiinae display a visible feature, their large oral sucker including a sphincter, no mention of Sphincteristomum in Mediterranean fishes, or fishes from adjacent seas, is in reviews from Janiszewska (1953) [13,14], Papoutsoglou (1976) [15], Radujkovic et al. (1989a) [16], Radujkovic and Raibaut (1989b) [17], Bartoli et al. (2005) [18].
And no mention of dissections of Balistidae, generally, is in this Sea. Yet, according to Tortonese in Hureau and Monod (1973) [10] Balistidae live in Mediterranean, Adriatic, Black Sea, European North Atlantic Ocean from Madeira to United Kingdom, African South Atlantic Ocean, from Angola to Morocco.
Probably, in Mediterranean, Balistidae neither fished out, nor dissected, have unknown their Digenean fauna.
Life history of Sphincteristomum remains unknown. The stomach of our dissected P. erythrinus contained leftover of shrimps. It suggested that these arthropods be second hosts of Sphincteristomum and could content their larval stages.
Live span of S. mediterraneae in P. erythrinus. The simultaneous presence of juvenile, ovigerous and senile, Sphincteristomum in P. erythrinus the few number of eggs in the worms, seems to suggest a short life span of Sphincteristomum in Sparidae, an imperfect adaptation of these parasites towards this family-hosts. Is the seasonal large number of parasitized Pagellus, a sign of a quick spawn in hosts storages or shifts?
Relationships between Schistorchiinae, Apocreadiinae and Postporinae. Apocreadiinae and Postporinae have a typical oral sucker devoid of sphincter, devoid of lobe. They have two typical blind caeca, devoid of ani. Conversely, Schistorchiinae have a peculiar oral sphincter, an oral sucker either devoid of lobe or displaying lobes. They have caeca ending into two ani. Schistorchiinae appear more recent than Apocreadiinae and Postporinae.
NOTES