Adaptability and recovery capability of two maize inbred-line foundation genotypes, following treatment with progressive water-deficit stress and stress recovery ()
1. INTRODUCTION
Plants usually experience fluctuating water supply during their life cycle because of variation in rainfall. Plants with a stronger recovery capacity have a greater chance of survival after stress recovery. However, plant responses to water-deficit stress [1,2] and growth recovery [3,4] are complex and differ with intensity of water shortage and crop genotype. Therefore, it is essential to understand the physiological and/or morphological processes that occur in the guard cells during progressive water-deficit stress and stress recovery [3].
Plant responses to water-deficit stress have been studied extensively, including biochemical and physiological processes as well as some molecular events [4-6]. Drought adaptation is related to minimization of water loss by control of the stomatal aperture in plants [7], and to maintenance of strong root water-uptake efficiency in wheat [8], which may be helpful to sustain a high leaf relative water content (RWC) or leaf water potential. In addition, many researchers reported that an increase in stomatal density and a decrease in stomatal length may enhance the adaptability of a plant to drought [9].
Maize (Zea mays L.) is an important crop worldwide. Its productivity is greatly constrained by water-deficit stress and genotypic factors. Successful plant breeding depends on the judicious choice of foundation genotypes from which to create and select desirable recombinants. Substantial research is needed to evaluate the adaptability of the improved genetic materials to particular environmental conditions [10]. It is desirable to identify genotypes that not only withstand greater levels of drought but also perform well after stress recovery. Two maize inbred lines, the foundation genotype Y478 and its derived line Z58, are widely used to breed novel maize cultivars in China, and the privious work showed foundation genotypes inbreed line Z58 showed eminent drought resistantce compared with that of inbreed line Y478 (data not showed). To the best of our knowledge, little information is available on physiological and/or morphological processes that occur in maize germplasm during progressive water-deficit stress and stress recovery.
In the present study, we investigated morphological responses, leaf micromorphology, gas-exchange parameters and chlorophyll fluorescence to compare the adaptability and recovery capability of two maize inbred-line foundation genotypes, following treatment with progressive water-deficit stress and stress recovery.
2. MATERIALS AND METHODS
2.1. Plant Materials and Treatment
The maize inbred line Y478 was used as a parent to breed a series of novel hybrid cultivars, which were widely cultivated in the Huang-Huai coastal region of China. During this process, a variant plant was observed by Zhang in 1988, and was inbred using a pedigree method for seven generations of selection. This novel inbred line was designated as Z58 in 1995 and possessed superior agronomic traits related to drought tolerance, disease resistance and yield compared to Y478 [11]. In the present study, seeds of Y478 and Z58 were surfacesterilized for 20 min in 75% ethanol, rinsed five times in sterilized distilled water, and germinated at 28˚C in sterilized wet river sand. Five days later, the residual embryo was excised from the seedlings, which were transferred to vigorously aerated, hydroponic tanks that contained Hoagland’s nutrient solution and cultured in growth chambers at 25˚C - 28˚C with a 12 h photoperiod and 60% - 80% relative humidity. During the growth period, the nutrient solution was replaced every 3 days. At the three-leaf stage, the seedlings were subjected to progressive water-stress treatment by sequential transfer at 24 h intervals to nutrient solution supplemented with 10%, 15% or 20% PEG 6000, at 24, 48 and 72 h, respectively. After treatment with 20% PEG 6000 for 24 h, seedlings underwent 72 h stress-recovery treatment by culture in nutrient solution that lacked PEG 6000. The control seedlings were cultured in nutrient solution that lacked PEG 6000 throughout the experiment. Collection of tissue samples and measurements were conducted at the same time (10:00) daily to reduce the impact of circadian variation.
2.2. Growth Parameters and Relative Leaf Water Content
Relative shoot growth rate (RSGR) of the aboveground tissues of the seedlings was estimated as described by Hilbert et al. [12]. Measurement of the leaf RWC followed the method described by Wang et al. [13]. Root: shoot ratio was calculated as the root dry weight per above-ground biomass dry weight. Root efficiency was defined as the full biomass divided by dry root weight [8].
2.3. Leaf Sampling and Microscopic Analysis
The harvested leaf samples were immediately fixed with 25% glutaraldehyde solution in 0.1 M phosphatebuffered saline (PBS; pH 7.2), rinsed three times with PBS (5 min each), dehydrated in a graded ethanol series, and vacuum-dried. After coating with gold, the samples were examined with a HITACHI EDAX S-3400N scanning electron microscope. Subsequently, three microscopic fields per replicate were randomly selected. Microphotographs were taken of each region with a digital camera. Stomatal density was calculated as the number of stomata per unit leaf area [14]. Stomatal area corresponded to the area inside the perimeter of two guard cells [15]. Stomatal size was defined as the length in micrometers between the junctions of the guard cells at each end of the stoma, and indicates the maximum potential opening of the stomatal pore, but not the aperture of opening that actually occurs.
2.4. Gas-Exchange Parameters and Hlorophyll Fluorescence
Stomatal conductance (gs), net photosynthetic rate (A), transpiration rate (E), and intercellular CO2 concentration (Ci) were measured with a portable LI-6400 photosynthesis system (Li-Cor, Lincoln, NE, USA). The chamber conditions were 28˚C, CO2 concentration of 385 ± 5 μl·l−1 and photosynthetic photon flux density of 1000 μmol·m−2·s−1.
Measurements of chlorophyll fluorescence parameters were carried out in situ on attached leaves using a portable, pulse-modulated fluorometer (PAM-2000, Walz, Effeltrich, Germany). The maximum efficiency of photosynthetic energy conversion of photosystem II (PSII) was determined by the ratio of variable to maximum fluorescence of dark-adapted leaves, i.e. Fv/Fm = (Fm – F0)/Fm, where Fm, Fv, and F0 are the maximum, variable and initial fluorescence, respectively. Samples were darkadapted with properly constructed leaf-clips for 20 min, which was found to be sufficient time to allow complete reoxidation of the PSII reaction centers and to ensure that all energy-dependent quenching was relaxed. To evaluate physiological responses to the water-deficit treatment, measurement of potential quantum yield (Fv/Fm) and effective quantum yield (ΔF/Fm′) of PSII were recorded in response to progressive water-deficit stress.
2.5. Statistical Analysis
Differences in leaf gas exchange parameters and leaf water potential among the two maize inbred-line foundation genotypes were tested by ANOVA. The values of leaf exchange parameters presented are the mean of 15 replicates of three samples, whereas data for other parameters are the mean of three replicates. The results are expressed as mean ± standard deviation (SD). Differences were considered significant at P = 0.05. The means of all parameters were compared with Duncan’s multiple range test at P = 0.05 using SPSS 13.0 software (SPSS, Chicago, IL, USA). To analyze relationships between the parameters recorded, linear regression and curve estimation were analyzed using a probability value of 0.05 as the significance threshold.
3. RESULTS
3.1. Morphological Responses to Progressive Water-Deficit Stress and Stress Recovery
3.1.1. Leaf Relative Water Content
Under well-watered conditions, the leaf RWC showed no significant difference between the two maize genotypes (Figure 1(a)). Under progressive water-deficit stress conditions, the leaf RWC was significantly higher in the derived line Z58 than in the foundation genotype Y478. The leaf RWC of Y478 declined more sharply with the duration of water-deficit stress than in Z58; the RWC decreased by 14.6% in Y478 and 10.2% in Z58 after 48 h of water-deficit stress, relative to the RWC of the control, and decreased further after 72 h of water-deficit stress (17.4% and 10.7% reduction for Y478 and Z58, respectively). After 72 h of stress recovery, the leaf RWC of Y478 increased to 86.2% of the level of the control, whereas the RWC of Z58 increased to 98.8% of the control.
3.1.2. Relative Shoot Growth Rate
The RSGR for Y478 was significantly higher than that of the derived line Z58 under the non-stressed condition (Figure 1(b)). Although the RSGR of the two genotypes showed the same decreasing trend during progressive water-deficit stress, the RSGR of stressed Y478 seedlings was significantly lower than that of Z58 seedlings throughout the experimental period. After 72 h of recovery from water-deficit stress, the derived line Z58 showed strong recovery in RSGR to 113.5% of the control RSGR, whereas the RSGR of Y478 seedlings recovered to 66.3% of that of the control.
3.1.3. Root:Shoot Ratio
The root:shoot ratio of non-stressed control seedlings was significantly different (P < 0.05) between the two genotypes (Figure 1(c)). The control Z58 seedlings had a lower root:shoot ratio than Y478 seedlings. The root: shoot ratio of both genotypes was higher under progresssive water-deficit stress, relative to that of the controls; the root:shoot ratio increased by 20.7%, 35.9%, and 38.9% for Z58 and 7.4%, 16.4%, and 6.0% for Y478 after 24, 48 and 72 h of water-deficit stress, respectively, relative to the ratios of the controls. After 72 h of stress recovery, the root:shoot ratio of Y478 seedlings increased to 103.8% of the value for the control, and that of Z58 seedlings increased to 121.7% of the value for the control.
3.1.4. Root Efficiency
The derived line Z58 showed markedly higher root efficiency than the foundation genotype Y478 in the nonstressed controls (Figure 1(d)). Root efficiency did not differ significantly between the two genotypes after 24 h of water-deficit stress. Root efficiency was reduced by 9.2% and 12.6% for Y478 and Z58, respectively, relative to the controls after 48 h of water-deficit stress. A significant difference between genotypes was observed after 72 h of water-deficit stress, at which time the root efficiency of Z58 was higher than that of Y478. After 72 h of stress recovery, root efficiency had increased to 102.1% and 89.3% of the levels of the non-stressed control for Y478 and Z58, respectively.
3.2. Leaf Micromorphology
3.2.1. Stomatal Area
Under non-stressed conditions, Z58 showed a higher stomatal area than that of Y478. A significant decrease in stomatal area for Z58 was observed after 24 h of waterdeficit stress, whereas the decrease was non-significant for Y478. A significant decrease was observed for Y478 with increasing duration of water-deficit stress. After 72 h of stress recovery, the stomatal area of Y478 and Z58 reached 47.4% and 37.9% of values for the control plants, respectively (Figure 2(a)).
3.2.2. Stomatal Density
Under the non-stressed condition, Z58 leaves showed a higher stomatal density than leaves of Y478. A significant decrease in stomatal density, relative to the nonstressed control, was observed for Y478 during waterdeficit stress and after 72 h of stress recovery. No significant difference was observed for Z58 after 48 h of water-deficit stress, but the difference was significant after 72 h of water-deficit stress and after stress recovery for 72 h (Figure 2(b)).
3.2.3. Stomatal Size
Y478 showed a significant decrease in stomatal size after 24 h of water-deficit stress, and a similar trend was observed with continued water-deficit stress and after stress recovery for 72 h. However, a significant decrease in stomatal size for Z58 was observed after 72 h of water-deficit stress, and a slight increase occurred for Z58 after 72 h of stress recovery (Figure 2(c)).
 (a) (b) (c)
(a) (b) (c)
Figure 2. Changes in the maize inbred lines Y478 and Z58 in stomatal area (a), stomatal density (b) and stomatal size (c) under progressive water-deficit stress and stress recovery (RW) for 72 h.
3.3. Physiological Responses to Progressive Water-Deficit Stress and Stress Recovery
3.3.1. Net Photosynthetic Rate
For both genotypes, A showed no significant difference between stressed and non-stressed seedlings after 24 h of water-deficit stress (Figure 3(a)). However, A was reduced by 20.4% and 16.8% for Y478 and Z58, respectively, after 48 h of water-deficit stress. After 72 h of stress, A had decreased by 37.1% and 27.8% for Y478 and Z58, respectively, relative to that of the non-stressed controls. After 72 h of stress recovery, A increased to 91.2% and 97.5% of the level of the non-stressed control for Y478 and Z58, respectively.
3.3.2. Stomatal Conductance
Stomatal conductance decreased significantly in both genotypes during progressive water-deficit stress, and differed significantly between the non-stressed and water -deficit-stressed seedlings (Figure 3(b)). The gs of Z58 was higher than that of Y478 under both non-stressed and water-deficit stress conditions. In Z58 the gs increased markedly after 72 h of stress recovery and was 91% of the value for the non-stressed control, whereas the gs of Y478 recovered only to 71% of the non-stressed control.
3.3.3. Transpiration Rate
In both genotypes, E showed a marked decrease under water-deficit conditions and continued to decline sharply with increased duration of water-deficit stress. In Z58 the value of E was always higher than that of Y478 under water-deficit stress, but both genotypes showed a marked increase in E after 72 h of stress recovery (Figure 3(c)).
3.3.4. Internal CO2 Concentration
The effect of CO2 on plant growth is mediated by water availability (Iker et al., 2009). After 24 h of waterdeficit stress, no significant difference in the internal CO2 concentration of stressed and non-stressed seedlings was observed for both genotypes (Figure 3(d)). The difference was significant for Y478, but non-significant for Z58, after 48 h of water-deficit stress. After 72 h of water-deficit stress, the internal CO2 concentration had decreased by 67.3% and 19.4% for Y478 and Z58, respectively. The internal CO2 concentration for Z58 was always higher than that of Y478 throughout the waterdeficit stress and stress recovery periods.
3.4. Chlorophyll Fluorescence Parameters
The effect of water deficit on Fv/Fm is shown in Figure 4(a). After 24 and 48 h of water-deficit stress, Fv/Fm values were between 0.82 and 0.84, and no appreciable differences between the stressed and non-stressed seedlings were observed for both genotypes. However, Fv/Fm values for both genotypes decreased sharply (by approximately 0.5% for Z58 and 6% for Y478) after 72 h of water-deficit stress relative to the non-stressed controls. The decrease in Fv/Fm value was recovered 72 h after removal of water-deficit stress. The Fv/Fm values for Z58 were slightly higher than those of Y478 during the course of the experiment.
Under the non-stressed condition, ΔF/Fm′ values were significantly higher for Y478 than for Z58 during the course of the experiment (Figure 4(b)). After 72 h of stress recovery, ΔF/Fm′ values recovered to 68% and 123% of the control values for Y478 and Z58, respectively.
No significant difference in non-photochemical quenching (qN) between the two genotypes was observed under the non-stressed condition. The values of qN for Y478 decreased significantly (P < 0.05) after water-deficit stress treatment for 48 and 72 h (Figure 4(c)). In contrast, qN values for Z58 remained relatively stable during the entire experiment, varying between 0.75 and 0.80, and were not significantly affected by the duration of waterdeficit stress and stress recovery. Curiously, Z58 seedlings showed slightly higher values of qN under waterdeficit stress and the values decreased to those of the control 72 h after the removal of water-deficit stress.
The capacity of photochemical quenching differed between the two genotypes under water-deficit stress. The difference of photochemical quenching (qP) for the two genotypes increased with the duration of water-deficit stress and decreased notably after 72 h of water-deficit stress compared with the respective control levels (Figure 4(d)). The qP value for Z58 recovered well, whereas that of Y478 recovered only slightly, after 72 h of stress recovery.
4. DISCUSSION
4.1. Growth Parameters
Growth is suppressed in plants subjected to progressive water-deficit stress. The utilization of leaf RWC as an indicator of the plant water status is common [16,17],
 (a)
(a) (b)
(b)
Figure 4. Changes in the maize inbred lines Y478 and Z58 in FV/Fm (a), quantum yield (b), non-photochemical quenching (qN; (c)) and photochemical quenching (qP; (d)) under progressive water deficit stress and growth recovery (RW) for 72 h.
and can differ significantly among cultivars exposed to the same period of water exclusion [18]. The leaf water status is related to grain yield and spikelet sterility in some cases [19]. In the present study, the adaptive responses to progressive water-deficit stress, in parameters such as leaf RWC, root:shoot ratio and root efficiency, varied between the two maize genotypes. The derived line Z58 maintained a higher leaf RWC under drought stress, which would be associated with its stronger ability to absorb water and the fact that it has more roots. In addition, the rate of reduction in leaf RWC during the progressive water-deficit stress treatment was correlated directly with specific leaf area (r = 0.78, P < 0.01) [20]. In the present study, we also observed a significant relationship (r = 0.63, P < 0.01) between RSGR and leaf RWC (Figure 5).
4.2. Correlation of Leaf Micromorphological Traits with Leaf Relative Water Content
Variation in leaf micromorphological traits is derived from genotypic and environmental factors. Micromorphological observations highlighted phenotypic variation among three Populus alba L. genotypes (6K3, 2AS11 and 14P11), of which the salinity-tolerant genotype 14P11 showed significantly smaller epidermal cells and higher stomatal density [15]. The present study indicated that the derived line Z58 could rapidly decrease stomatal area by closure of the stomatal aperture early in waterdeficit stress (24 h water-deficit stress) without a significant reduction in stomatal size, which could effectively decrease water loss. However, Y478 may reduce stomatal size by pore closure between the junctions of the guard cells at each end of the stoma. In a few studies, the modification of a single gene resulted in reduced stomatal aperture and stomatal density, and consequently increased water use efficiency [21]. A similar decreasing trend in stomatal density and stomatal area with increase-
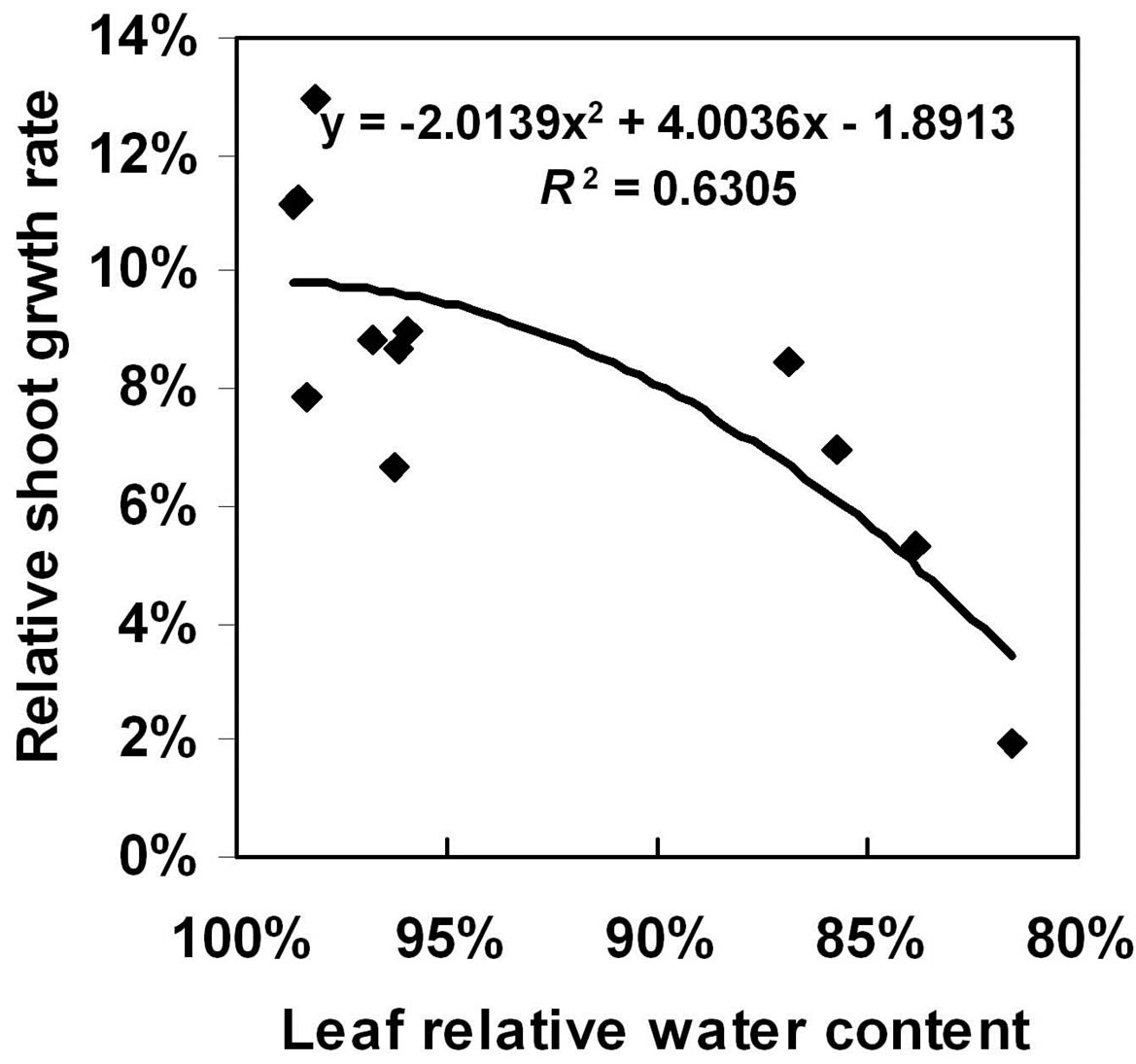
Figure 5. Relationship between relative shoot growth rate and leaf relative water content at the three-leaf growth stage in the maize inbred lines Y478 and Z58.
ing duration of water-deficit stress was observed for both maize genotypes. In addition, our results also showed that there was a non-linear response of stomatal density to leaf RWC, described by a quadratic parabolic curve, with a maximum of 146.3 pores mm−2 when leaf RWC was 94.5% at an early stage of water-deficit stress (Figure 6). Similar results have been reported previously for rice [22] and Leymus chinensis [23].
4.3. Correlation of Gas Exchange with Leaf Relative Water Content
As reported in other studies, soil water stress may limit gas exchange owing to both stomatal and metabolic limitations [24,25]. However, soil water content is not always related with the leaf water content in genotypes that exhibit that differences in drought tolerance. In the present study, photosynthesis showed a parabolic curve response to leaf RWC, and the photosynthetic rate was a maximum of 12.94 μmol·CO2·m−2·s−1 when leaf RWC was 98.1% (Figure 7(a)). A similar quadratic parabolic curve response of gs to leaf RWC was observed, but gs was a maximum of 0.157 mmol·H2O·m−2·s−1 when leaf RWC was 93.8% at an early stage of water-deficit stress (Figure 7(b)). These findings showed that the maximum photosynthetic rate was not completely consistent with that of gs in relation to leaf RWC, which indicated that the photosynthetic capability of the maize genotypes was more dependent on the optimal gs than the maximum gs.
4.4. Correlation of Chlorophyll Fluorescence Parameters with Leaf Relative Water Content
Measurement of chlorophyll fluorescence by probebased systems has been utilized for non-invasive analyses of stress-induced (ozone, drought, and high CO2) perturbations to photosynthesis for several decades [26, 27]. A recent study showed that Fv/Fm declined rapidly

Figure 6. Relationship between stomatal density and leaf relative water content at the three-leaf growth stage in maize inbred lines Y478 and Z58.

Figure 7. Relationships among net photosynthetic rate (a) and stomatal conductance (b) with leaf relative water content at the threeleaf growth stage in the maize inbred lines Y478 and Z58.
during increased drought stress and could serve as an indicator of the latter phase of drought and subsequent loss of viability [28]. The present study indicated that there was a non-linear response of Fv/Fm to leaf RWC, which was described by a quadratic parabolic curve with the maximum Fv/Fm of 0.833 when leaf RWC was 93.4% at an early stage of water-deficit stress (Figure 8(a)). The Fv/Fm value declined rapidly with a gradual decrease in leaf RWC. Similar findings have been reported previously in Arabidopsis [28]. After leaf RWC has started to decrease, one would expect that qP would also decrease (Figure 8(b)), which shows a non-linear response to leaf RWC. In addition, our results also showed that the leaf electron transport rate decreased non-linearly (y = −17.564x2 + 70.968x − 34.115; R2 = 0.7112) with decline in leaf RWC (Figure 8(c)).
4.5. Growth Recovery
Li et al. [4] indicated that fine transcriptional coordination between maize leaves and roots is one factor that constitutes a strong capability for growth recovery from water deficit. The capability for growth recovery varied between genotypes. The derived line Z58 was selected from among inbred variants of Y478, and is character-

Figure 8. Relationships among Fv/Fm (a), photochemical quenching (b) and electron transport rate (c) with leaf relative water content at the three-leaf growth stage in the maize inbred lines Y478 and Z58.
ized by low root biomass, a higher root:shoot ratio and higher root efficiency, which contribute to the higher leaf RWC and RSGR of Z58. Leaf micromorphology was used as an indicator of drought tolerance, and many studies [29,30] have confirmed that stomatal density is positively correlated with drought tolerance. Stomatal size also plays a critical role in regulation of the partial opening of stomata after stress recovery. In addition, the differences in leaf micromorphological traits (stomatal area, size, and density) were consistent with the changes in physiological parameters, and may contribute to the derived line Z58 possessing an increased ability for growth recovery from drought stress.
5. ACKNOWLEDGEMENTS
This research was supported by the National Natural Science Foundation of China (No. 31160287), Guangxi Natural Science Foundation (No. 2011GXNSFB018052), the Development Program for Guangxi Science and Technology Research (No. Guikegong 10100005-4).