1. Introduction
Dissolution processes have been extensively utilized to separate valuable minerals from their ores. Several researches [1-5] have been carried out on either the dissolution or both the dissolution and kinetic study of iron ore in different reagents such as HCl, H2SO4, oxalic acid et cetera. It was observed that the dissolution of iron ore or even the extraction of iron from an ore containing it increases as the concentration, temperature, time and stirring speed increases but decreases as the particle size increases due to its decreasing surface area [3-5]. Baba et al. [1,2] observed that iron ore dissolves easily and more efficiently in HCl than in H2SO4 or HNO3 and it was believed to be as a result of ferric-chloride complexes formation. It therefore required less energy for the reaction to occur comparing the activation energies of 13.63 kJ/mol in HCl and 38.29 kJ/mol in H2SO4 respectively. The kinetic study of these dissolutions reaction processes were carried out using the shrinking core models to interpret the processes involved in either the leaching or dissolution [1,3,4,6]. During the leaching process, three major steps occur; diffusion or mass transfer through the liquid film surrounding a solid particle, chemical reaction on the surface of the un-reacted core, and diffusion through the ash/inert solid layer. The slowest between these steps is considered the rate determining step. The model first developed by Yagi and Kunii [6-8] helped to derive the rate determining step from the experimental data using the equations below:
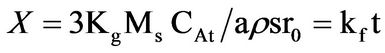 (1)
(1)
 (2)
(2)
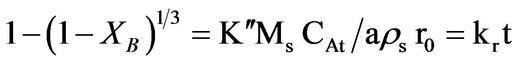 (3)
(3)
where Kf, Kd, and Kr are the rate constants for the liquid film diffusion, ash/inert solid layer diffusion, and surface chemical reaction respectively. XB is the fraction reacted, MS is the molecular weight of the solid, CA is the concentration of the dissolved lixiviant A in the bulk of the solution, “a” is the stoichiometric coefficient of reagent in the leaching reaction, r0 is the initial radius of the solid particle, ρs is the density of the solid, D is the diffusion coefficient in the porous product layer, Kg is the mass transfer coefficient between fluid and solid particle, “K” is the first-order rate constant for the surface reaction and t is the contact time. This research work is intended to study the dissolution of iron ore in a 3:1 HCl-HNO3 system (similar to aqua regia) which is a well known reagent for dissolving un-reactive metal minerals such as gold, silver, et cetera and also the kinetic evaluation using three different shrinking core models (SCM). So far, there is no data to this effect viz-a-viz the use of Nigerian iron ore is concerned.
2. Materials and Methods
2.1. Materials
Iron ore from the Toto Muro iron ore deposit in Nasarawa State of Nigeria was used for this study. Chemical analysis of the sample ore was done with Leeman Model of Inductively coupled plasma-optical emission spectrophotometer. Different particle sizes (<75 µm, 75 µm, 125 µm and 150 µm) were obtained with the use of 8 inch diameter size mechanical sieve shaker. Distilled water and analytical grade chemicals (BDH) were used as provided to prepare all the solutions.
2.2. Methods
Particle sizes of less than 75 µm sample of the iron ore was used for the experiment at a constant concentration HCl-HNO3 solution and a fixed temperature of 331 K but at different contact time. 1.0 g of the given particle size sieve sample was weighed and then transferred into a 250 ml beaker containing 15 ml of 8 M HCl and 5 ml of 8 M HNO3 and made to 100 ml with distilled water. The mixture was agitated manually with a glass stirrer. The mixture was stirred and heated to 331 K for various contact times of 20, 30, 60 and 100 min. At the end of each period, the solution was cooled and filtered into a 100 ml standard flask and analyzed.
The same procedure was also repeated for different acid (HCl-HNO3) concentrations of 4 M, 2 M and 1 M at 331 K for 20, 30, 60 and 100 min. This same procedure described above was repeated for 343 K and 353 K for 20, 30, 60 and 100 min with 8 M acid concentration. The following particle size fractions were examined 75 µm, 125 µm and 150 µm at a fixed temperature of 353 K for 30 min with 8 M acid concentration. The kinetics of the ore dissolution was also investigated for the effects of temperature, acid concentration, particle size and contact time.
3. Results and Discussion
3.1. Chemical Analysis
The iron ore was analyzed chemically using the inductively coupled plasma-optical emission spectrophotometer and the results are shown in Table 1. The primary elements found in the sample included 62.10% Fe, 21.7% O, 11% Cu. Other elements such as Mn, Mg, K, Ca et cetera make up the 5.2% of the sample. From these analytical results, it is concluded that the ore was an iron ore.
3.2. Effect of Acid Concentration
The effect of 3:1 HCl-HNO3 solution concentration on the dissolution of the iron ore was studied using the following concentrations; 1 M, 2 M, 4 M, and 8 M. Figure 1 is the graphical presentation of the result. From the figure, it can be seen that the iron dissolution rate increases as the concentration of the HCl-HNO3 system increases from 1 M to 8 M at the same contact time. The optimum dissolution of 81% iron was achieved at the system concentration of 8 M.
3.3. Effect of Temperature and Time
The effects of temperature and contact time on iron dissolution were studied at varying temperatures (331 K, 343 K and 353 K) and at varying time (20 min, 30 min, 60 min, and 100 min). The results are shown in Figure 2. From Figure 2 it is observed that as the temperature increases, dissolution of the iron increased with tempera-

Figure 1. A graph of quantity of iron leached (%) vs contact time (min) with respect to concentration [particle size, <75 µm; temperature, 351 K; mass of ore, 1 g].

Figure 2. A graph of iron leached (%) vs contact time (min) with respect to temperature [particle size, <75 µm; conc., 8 M; mass of ore, 1 g].

Table 1. Elemental analysis of the iron ore sample.
ture and period of contact. When the temperature was increased from 331 K to 343 K at a particular period (20 min) only 2.5% increase in the quantity of iron leached was achieved. At temperature 331 K, there was 8.5% increase in the quantity of iron leached. Optimum temperature and period was found to be 353 K and 100 min respectively in which 88% of the iron was leached.
3.4. Effect of Particle Size
Table 2 shows the experimental data when different particle sizes were investigated at 8 M HCl-HNO3 solution, temperature of 353 K and contact time of 60 min. Four particle sizes were studied—<75 µm, 75 µm, 125 µm and 150 µm and from the data it was observed that the smallest particle size (<75 µm) gave the highest percentage of iron leached (87%) which is expected due to higher surface area for smaller particle size.