1. Introduction
With the development of economy, the consumption of industrial cooling water is increasing rapidly. Because of evaporation, concentration, temperature rise and other reasons in the process of operation, dissolved salts will be separated out from cooling water and it can adsorb in pipe wall and equipment in the form of scale, the metal surface may form electrochemical corrosion because of irregularity, both of them will affect the equipment, service life of pipelines and use efficiency. Therefore people have to pay more attention to the treatment of the circulating cooling water [1,2].
Recently, dosing reagents into circulating cooling water is the most common method, and through the role of the pharmacy, the corrosion and scaling problem in the pipes will be solved effectively [3-6]. Now, phosphorus products are widely used in cooling water system. Because they can easily cause environmental pollution, an intense research effort is being undertaken to look for the replacement of phosphorus products by more environmentally friendly products [7-12]. The previous work has shown that Chitosan is a natural polymer material which has good resistance scale effect; benzotriazole and zinc salts both have corrosion inhibition effect.
The present work aims to study the performance of a new corrosion and scale inhibitor mixed by chitosan (CTS), polyacrylic acid, benzotriazole (BTA) and zinc salt in cooling water. The best formula has been found and it has obvious economic and environmental benefits.
2. Experimental
2.1. Materials and Instruments
Test specimens were 50 × 25 × 2 mm sheets prepared from A3 carbon steel. The exposed surfaces degreased with acetone. The corrosion products were eliminated by HCL at 10% for 3 minutes before tested.
The chitosan was made from shrimp. After removing impurities, cleaning the selected shrimp by water, soaking it into 10% hydrochloric acid for 3 days in order to remove the calcium in it. After filtration and washing, placed it into 10% NaOH, heated it to boiling for 3 hours, oxidated it with 4% KMnO4 for 2 hours, then added 0.2% NaHSO3 in order to fade the colour of KMnO4 completely. Finally dry it to get white flakes of chitin. Placed 5 g chitin into a three-necked flask equipped with 50 mL 50% sodium hydroxide solution, keeping the temperature 100˚C, stirring speed 50 r/min, stirring time 50 min under the conditions of the deacetylation reaction, then filtration, washing and drying to get chitosan products, finally taking the chitosan products to prepare CTS working fluid of 0.02%.
2.2. Experimental Methods
2.2.1. Rotating Hanging Plate Experiment
Corrosion inhibition efficiency was determined by weight loss of the tested specimens using rotating hanging plate method [13], afterward, the instruments used was RCC-type I rotate hang piece of test, the experiment was achieved in laboratory configuration water (Table 1) at temperature 45˚C ± 1˚C, time 72 h and rotation speed 75 r/min. The corrosion rate X and corrosion inhibition rate η were calculated respectively by Types (1) and (2):
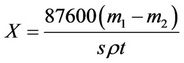 (1)
(1)
where m1 and m2 were the quality of A3 carbon steel before and after the test respectively. s is the surface area, cm2; ρ is the density. g/cm; t is the experimental time, h
 (2)
(2)
where X0 and X1 were the corrosion rate without and with the corrosion and scale inhibitor.
2.2.2. Static Scale Inhibition Experiment
Scale inhibition efficiency was tested by “Calcium carbonate deposition method” [14]. The principle was to heat water samples with and without the scale and corrosion inhibitor, then determined the concentration of Ca2+ and calculated the inhibition rate. The experiment was achieved at temperature 80˚C ± 1˚C and time 10 h. Scale inhibition rate η was calculated by Type (3):
 (3)
(3)
where ρ0 and ρ1 were the Ca2+ concentration without and with corrosion and scale inhibitor ,while ρ2 was Ca2+ concentration before experimenting.
3. Results and Discussion
3.1. Best Formula of the New Phosphate-Free Corrosion and Scale Inhibitor
Adequate experimental studies have been made to determine the preliminary formula. In this paper, Table 2 shows the four factors and their levels .The final formula would been determined by further experiments.
The best formula would be determined through orthogonal experiment used calcium carbonate deposition method (GB/T16632-2008). The initial Ca2+ concentra-
tion was 240 mg∙L−1, scale inhibition rate was calculated by Type (3). Table 3 shows the importance of factors as polyacrylic acid > CTS > BTA > zinc salt, the best formula was B3C2A2D2.
3.2. Corrosion Inhibition Performance Analysis
Table 4 contains the corrosion inhibition efficiency of A3 carbon steel samples in colling water with 0PPM, 10 PPM, 20 PPM, 30 PPM and 40 PPM corrosion and scale inhibitor .In all cases the typical behavior was observed, corrosion inhibitor rate of the phosphorus—free corrosion inhibitor increased as the concentration elevated. The corrosion inhibition rate could reach 96.14% when the dosage was 30 PPM .