Process for the Obtention of Coumaric Acid from Coumarin: Analysis of the Reaction Conditions ()
1. Introduction
Coumarin, a source for coumaric acid, was identified in 1820 and 1868, was synthesized in a laboratory for the first [1]. It is a pleasant smelling compound which gives a characteristic odor to hay. Other simple coumarins also possess characteristic smells sometimes exploited in perfumery [2].
Coumarin derivatives also have diverse biological properties, such as enzyme inhibition, hypotoxicity, as well as, carcinogenic, anticoagulant or antibiotic action [3]. Also, some are used as dyes given their efficient light emission properties, high stability, and ease of synthesis [4].
The bicyclic ring system of chromenes, like coumarin, has inspired a number of different synthetic approaches [5,6].
The purpose of this research was to determine the best process conditions (time, temperature, NaOH concentration and solvent, and reaction atmosphere) to obtain coumaric acid from basic hydrolysis of coumarin.
Several processes have already been developed for the conversion of coumarin into coumaric acid. Scheme 1 shows one of these methods; where coumarin (1) converts into o-coumaric acid (2) via reflux with sodium ethoxide during four hours; then, the mixture is diluted with water, removing most of the solvent with vacuum, and neutralizing the residue with concentrated hydrochloric acid [7,8].
Other method (Scheme 2) establishes that strong nucleophiles, such as hydroxide ions, open the coumarin ring as a result of an attack at C-2 [1,9]. Initially, the double bond is cis, this product is known as coumarinic acid (3). Acidification of the solution at this stage is followed by rapid cyclization to reform coumarin (even under slightly basic conditions). However, on prolonged contact with the base, the cis-acid slowly isomerizes into trans-isomer (4) (known as coumaric acid). Acidification at this step allows isolating free hydroxy-acid.

Scheme 1. Reaction to obtain coumaric acid from coumarin.

Scheme 2. Reaction to obtain coumaric acid from coumarin with strong nucleophiles.
Other authors point out that when coumarin is treated with ethanolic sodium ethoxide at reflux, the lactone ring is opened (Scheme 3) to give 2-(ethylpropenoate)-phenoxide (5). Subsequent treatment of (5) with water and evaporation of the solvent brought about saponification of the ester and, with careful acidification, produces ocoumaric acid [10-12].
Other technique [13-15] establishes that when coumarin is heated with a sodium bisulfite solution, it dissolves completely and a sulfonate compound (6) is formed (Scheme 4), which contains the lactone ring intact. Alkali splits off the sulfo group, liberating coumarin, which recombines with neutral sulfite and forms a hydrocoumaric acid derivative. The hydrocoumaric sodium sulfonate (7) is treated with 50% potassium hydroxide solution and the mixture is evaporated to dryness in a water bath. The residue is then dissolved in water, and the cooled solution is acidified with hydrochloric acid. The precipitate obtained is coumaric acid.
2. Materials and Methods
2.1. Reaction
Coumarin (0.137 mol) was dissolved in a sodium hydroxide solution on a PARR reactor. The reactor was purged for about 15 to 20 minutes, to maintain the required reaction atmosphere.
When the reaction was finished, the reaction mixture was cooled into a flask, which was set into an ice bath to reach 4˚C. Afterwards, concentrated hydrochloric acid was slowly added, until a white precipitate appeared at pH 5.
The precipitate, mostly coumaric acid, was filtrated and washed with cold water. Then, more hydrochloric acid was added to the liquid phase until a second precipitate appeared. Used reagents like H2O, HCl, and NaOH were of AR grade.
2.2. Purification
The precipitate, containing coumaric acid and coumarin, was placed in a solid-liquid extraction system (Soxhlet) with chloroform. Coumarin was extracted and coumaric acid remained in the extraction thimble.
2.3. Analysis methods
The chemical composition of the products was determined by Gas chromatography. The sample was heated from 50˚C up to 100˚C at 20˚C/min, then kept at 100˚C for 3 minutes and heated again to 300˚C at the same heating rate. Afterwards, the sample was kept at 300˚C, for 3.5 minutes. The equipment was flushed with helium at 2.0 mL/min and at pressure of 15.5 psi.
3. Results and Discussion
3.1. Effect of Reaction Atmosphere
The effect of reaction atmosphere was studied for coumarin reaction with 20% of NaOH on water at constant temperature of 160˚C and 1 h time period.

Scheme 3. Reaction to obtain coumaric acid from coumarin using ethanolic sodium ethoxide.
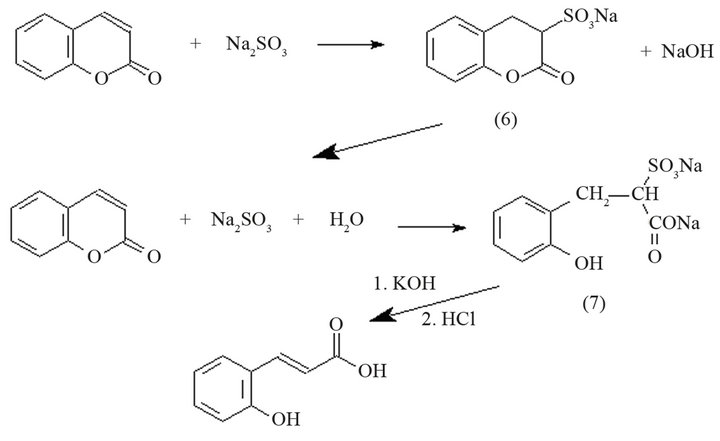
Scheme 4. Reaction to obtain coumaric acid from coumarin using sodium bisulfite.
Figure 1 shows the necessity of using an inert atmosphere. When the reaction is carried out without eliminating air from the medium, the mixture favors oxidation reactions to give by-products, which complicate the handling of material and the yield decreases (Table 1). Therefore, it was decided to pass an inert gas for 20 minutes to eliminate the air from the medium.
3.2. Effect of the Reaction Temperature
The effect of the reaction temperature on the conversion of coumarin into coumaric acid is displayed in Figure 2, for 1 h time period, 20% NaOH aqueous solution, and in a helium atmosphere. The amount of reaction yield is shown in Table 2.

Table 1. Effect of the reaction atmosphere.
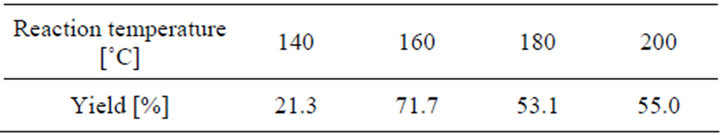
Table 2. Effect of the reaction temperature.
Below 120˚C, there is not conversion of coumarin into coumaric acid, and above 180˚C by-products are obtained (Figure 3). The peaks found at 5.77 min and 6.21 min correspond to phenol and 2-hydroxybenzaldehyde, respectively. These two compounds are due to the oxidation of the product, and the presence of them can be observed when the final reaction mixture has an orange color instead of a yellow one. When they are contained in the final reaction mixture, acidification gives a new tacky material, which complicates the handling of the product, as well as the purification process. The temperature at which the best yield is obtained is 160˚C.
3.3. Effect of the Sodium Hydroxide Concentration
The effect of NaOH concentration on coumaric acid production was studied using different concentrations within the range of 10% - 25%, at a constant temperature of 160˚C, and in a helium atmosphere. Results, shown in Figure 4 and Table 3, reveal that the best yield of coumaric acid was obtained at 20 wt.% of NaOH in the reaction mixture. An increase in the caustic soda content produces a variation in the yield, since by-products such as phenol may appear and complicate the purification process. Thus, 20 wt.% sodium hydroxide solution addition is considered to be the optimum concentration.

Figure 1. Effect of the reaction atmosphere.
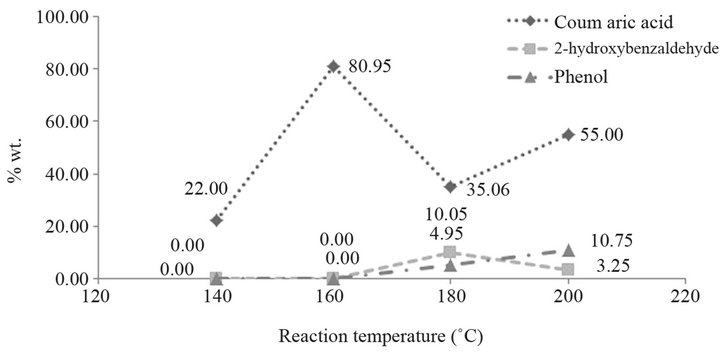
Figure 2. Effect of the reaction temperature.
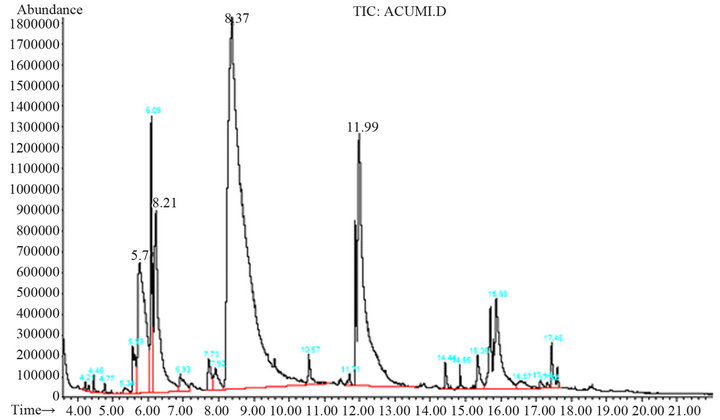
Figure 3. Chromatogram of by-products obtained when the reaction was carried out at temperatures above 180˚C.

Figure 4. Effect of the sodium hydroxide concentration.

Table 3. Effect of the sodium hydroxide concentration.
3.4. Effect of the Reaction Time
The reaction of coumarin was carried out with 20% of NaOH aqueous solution, at a constant temperature of 160˚C, in a helium atmosphere, and during a period of time from 0.5 to 1.5 h.
Table 4 shows that appropriate time to obtain the best yield is 1 hour. Below one hour the yield decreases and above it, oxidation and esterification reactions are favored,