1. INTRODUCTION
To describe the physical manifestations of lamotrigine toxicity presenting as skin rash and angioedema.
2. CASE REPORT
A 23 years old Hispanic female presented to Lincoln Medical and Mental Health Center with itchy rash affecting her face, body, and swelling of her eyelids and lower lip, after taking approximately 20 pills of lamotrigine in an apparent suicide attempt. She denies SOB, cough, fever, palpitation, headache or blurry vision. She has history of bipolar disorder and used to be on lamotrigine 25 mg bid for many months. No other significant medical history. No history of allergies. Patient uses Alcohol daily and Marijuana sometimes. Physical exam show mild edema in eyes lids, few erythematous edematous papules on cheeks, bilateral upper extremities (Figures 1 and 2), lower extremities, chest, abdomen and back. The rest of physical exam was normal. Blood work normal, urine toxicology was positive for Marijuana. EKG showed prolonged QT interval (Figure 3). Steroids and anti-histamine started which improved her symptoms.
Dermatology assessment: Urticarial with angioedema, may be related to lamotrigine overdose.
3. DISCUSSION
In our case, the patient developed mild angioedema and urticaria, 12 - 24 hours, after she took approximately 500 mg of lamotrigine. Lamotrigine is a new antiepileptic medication approved by the US Food and Drug Administration as adjunctive treatment of partial seizures in adults and children, as well as for adjunctive therapy for primary generalized tonic-clonic seizures and Lennox-Gastaut syndrome [1,2]. It is also used as first line medication for bipolar disorder maintenance treatment [3]. Common side effects of lamotrigine at regular dose include nausea, vomiting, chest pain, peripheral edema, insomnia, somnolence, impaired coordination, dizziness, ataxia, irritability, rash, dermatitis, dysmenorrhea, increased libido, dyspepsia, nystagmus, abnormal vision, and in rare cases have been reported to cause multiorgan hypersensitivity reactions, life-threatening skin rashes, CNS depression, aseptic meningitis [4-7]. Overdose of lamotrigine usually causes drowsiness, lethargy, nausea, vomiting, ataxia, dizziness, tachycardia, coma, respiratory depression. There is report of a case in which lamotrigine caused anticonvulsant hypersensitivity syndrome [8,9]. The incidence of urticaria, periorbital and lip edema, immediately after Lamotrigine overdose in our patient, made us suspect overdose as the cause of the symptoms.
4. CONCLUSION
Lamotrigine overdose may manifest as angioedema, and
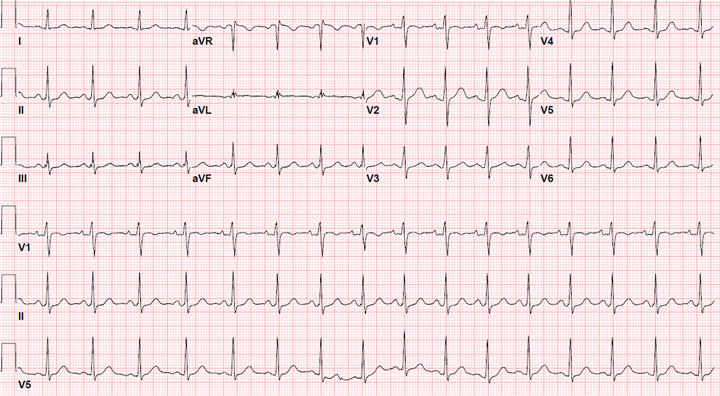
Figure 1. EKG show sinus tachycardia with prolong QT intervals.
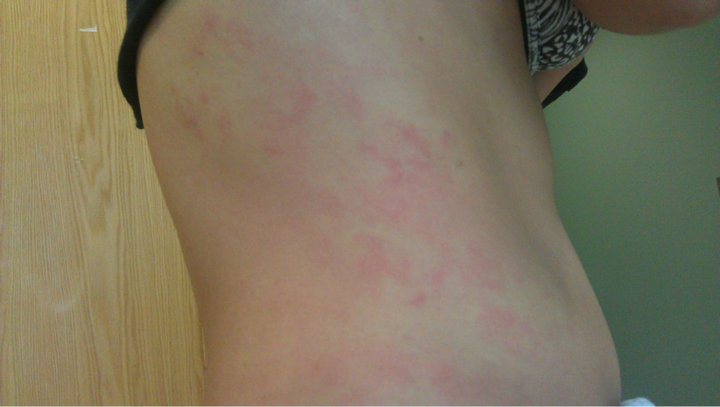
Figure 3. Rashes in medial aspect of left lower extremity.
urticaria. Health care providers should be aware about these complications and able to medically optimize the patient as needed.
NOTES
Author’s Contributions: Mohammad Alkayem, MD: first author, wrote whole the case, took the consent and the Images, prepared the article for publication and sent it to OJIM. Hussein Assallum, MD: second author, reviewed and revised the article.
Conflict of Interest: Null.
#Corresponding author.