Protective effects of polar lipids and redox-active compounds from marine organisms at modeling of hyperlipidemia and diabetes ()
1. INTRODUCTION
Cardiovascular diseases and diabetes mellitus are leading causes of mortality in modern society (both in developing and highly developed countries). This is attributed to the increased proportion of elderly people, growth and prevalence of obesity and stresses of various etiologies [1,2]. Thus, searching for novel effective, low toxic and easily available tools of prophylaxis and additional therapy of diabetes mellitus and hyperlipidemia among natural sources of biologically active substances exhibit various mechanisms of protective effects and corrective impaired biochemical status of the body represents an important task for modern medicine.
There is evidence [3] that diabetes mellitus and atherosclerosis may be referred to some extent to inflammatory diseases. Reactive oxygen species (ROS) refer to group of small reactive molecules that include hydrogen peroxide (H2O2). ROS are a consequence of aerobic metabolism and react avidly with other molecules, cellular lipids, proteins, and nucleic acids. Low to moderate levels of ROS have been shown to contribute to impotent functions, such as cell differentiation, migration, senescence, growth, and apopotosis. In contrast, a variety of diseases, such as cardiovascular pathologies, diabetes, and cancer, are associated with elevated ROS levels [4].
This suggests that redox-active compounds (antioxidants) may also exhibit corrective effects on impairments of carbohydrate and lipid metabolism. Antioxidants are already used in medicine due to their ability to inhibit lipid peroxidation (LPO) in biomembranes, stabilize structure and function of cell membranes and therefore to optimize conditions required for homeostasis of cells and tissues of the body exposed to various pathogenic factors [4,5].
Biological activity of echinochrome A, a polyhydroxy naphthoquinone isolated from the flat sea urchin Scaphechinus mirabilis, is attributed to its antioxidant properties [5]. Echinochrome A is an acting compound of the cardioprotector preparation “Histochrome”, but its corrective properties in impairments of carbohydrate and lipid metabolism have not been investigated so far.
Rosmarinic acid, luteolin and its sulphate analogies are main components polyphenol complex from the sea grass. Zostera marina is designated from Luromarin. Luteolin and rosmarinic acid is a common polyptenolic compound that exists in many types of plants. Having multiple biological effects such as antiinflammation, antiallergy, anticancer, and many others, luteolin and rosmarinic acid functions biochemically as redox-active compounds, i.e. either an antioxidant or pro-oxidant. The boilogical effects of luteolin and rosmarinic acid could be functionally related to each other. Besides antioxidant and antiinflammatory activity rosmarinic acid and luteolin also act as activators of peroxisome proliferator-activated receptors (PPAR) [6,7], which are important regulators of lipid and carbohydrate metabolism [8].
In this study we have investigated the possibility of correction of experimental impairments of carbohydrate and lipid metabolism by a mixture of polar lipids from sea macrophytes (Ulva fenestrata, Sargassum pallidum, Zostera marina) and its combination with natural redoxactive compounds: the polyhydroxy naphthoquinone echinochrome A from the flat sea urchin Scaphechinus mirabilis and a polyphenolic preparation Luromarin prepared using the sea grass Zostera marina. The latter preparation contains rosmarinic acid, sulphated luteilin in sort monoand disulphates, and bioflavonoids (luteolin, apigenin, and others) in approximate percentage (45:45: 10), correspondingly. In Figure 1 represented chemical structures of tested redox-active compounds.
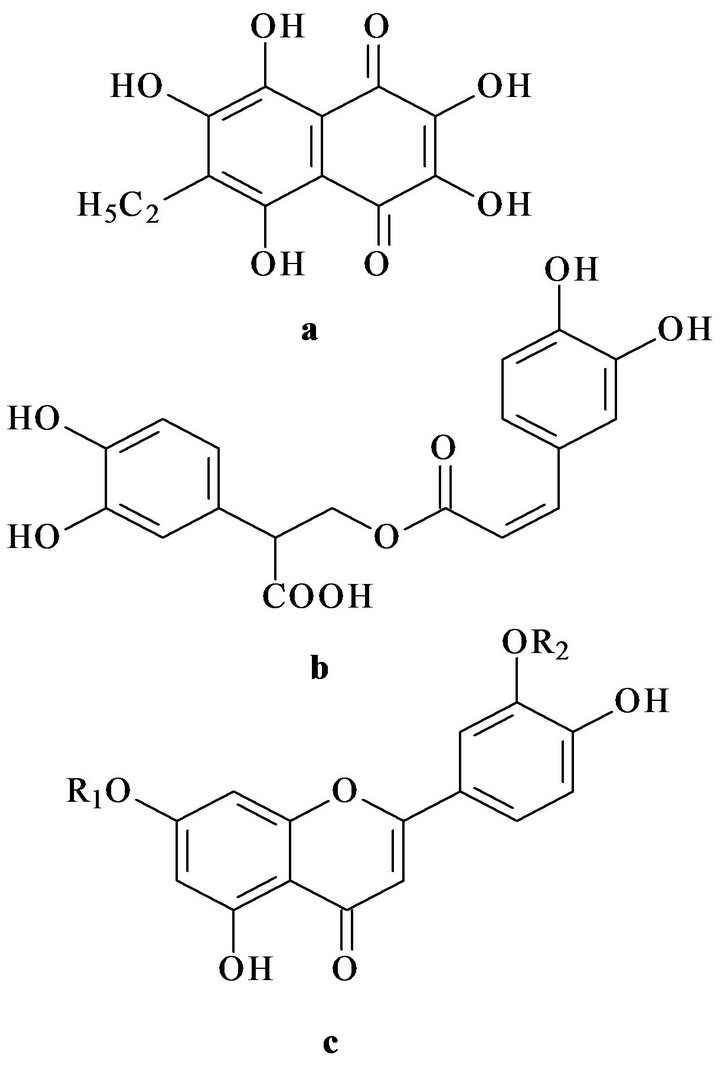
Figure 1. Chemical structures of echinochrome A (a), rosmarinic acid (b) and luteolin (c). R1 and R2—sulphate groups.
2. MATERIAL AND METHODS
2.1. Lipid and Antioxidant Isolation
A mixture of phosphorand glycolipids was obtained from the total lipid extracts of sea macrophytes Zostera marina, Ulva fenestrata, Sargassum pallidum, fished in the Sea of Japan during autumn periods of 2004-2005 at 20˚C (Kievka bay, Popov island). Total lipids were extracted by the method of Folch [9]. Crude lipid separation was performed by column chromatography. Fatty acid composition was analyzed as corresponding methyl esters using method of gas liquid chromatography [10].
The polyphenolic preparation Luromarin composed percentage from rosmarinic acid (about 45%), luteolin sulphates (about 45%) and bioflavonoids (luteolin, apigenin and ather (approximately 10%) was prepared from the sea grass Zostera marina as described in patent [11].
Echinochrome A was also isolated from the flat sea urchin Scaphechinus mirabilis by researchers of the Laboratory of Biotechnology of the Pacific Institute of Bioorganic Chemistry using the previously published method [12].
2.2. Animals
Investigations with the use of experimental animals were conducted in accordance with the rules of laboratory practice (GLP), the Order No. 267 of Ministry of Health of the Russian Federation of June 19, 2003 On Approval of the Rules of the Laboratory Practice and Instruction on Experimental (Preclinical) Study of New Pharmacological Preparations (2005). The test animals were kept in accordance with the rules accepted by the European Convention for the protection of vertebrates used for experimental and other scientific purposes (Strasbourg, 1986).
Female CBA mice (8 - 10 weeks old) is bred in vivarium of Pacific Institute of Bioorganic Chemistry. All animals were healthy, housed in five mice per cage at 20˚C. Water and a standart diet were available ad libitum.
2.3. Induction of Alloxan Diabetes and Hyperlipidemia
CBA mice (18 - 20 g) were used in experiments. Diabetes mellitus was modeled by intraperitoneal administration of alloxan (120 mg/kg in saline) to the overnightfasted animal’s according to published method with minor modifications [4,11]. Briefly, animals were subdivided into the following groups (n = 7 in each group): 1) intact mice; 2) diabetic mice (negative control); 3) diabetic mice treated during four days before and four days after (4 + 4) induction of alloxan diabetes.
Changes in carbohydrate metabolism induced by alloxan diabetes were determined on the 9th day after the beginning of the experiment in the glucose tolerance test (60 min after peroral administration of 4 g/kg glucose). Blood glucose was determined using a Satellite glucometer (Elta, Russia).
Hyperlipidemia was modeled by a single dose administration of Tyloxapol (Triton WR-1339; 40 mg/100 g of body weight) as previously described [4,11]. Animals were subdivided into the following groups (n = 7 in each group): 1) intact mice; 2) hyperlipidemic mice (negative control), 3) hyperlipidemic mice treated during three days before induction of hyperlipidemia. The mixtures of polar lipids (3 mg/kg) and bioantioxidants (5 mg/kg) were administered per os. At the end of experiments blood samples were taken for determination of the main parameters characterizing changes in lipid and carbohydrate metabolism and also aspartate amino transferase (AST) and alanine aminotransferase (ALT) activity using a biochemical analyzer (ROCHE, Switzerland).
2.4. Statistical Analysis
Statistical and graphical treatment of experimental data was performed using Microsoft Excel. Results were expressed as mean ± SD. Statistical differences were evaluated using paired Student’s t-test. Differences were considered as statistically significant at p < 0.05.
3. RESULTS AND DISCUSSION
GLC-analysis has shown (Tables 1 and 2) that in sea macrophytes Sargassum pallidum, Ulva fenestrata, and Zostera marina, the molar ratio ω-3/ω-6 PUFA is 1.3:1, 3.1:1, and 5:1, respectively.
Subsequent pharmacological studies have demonstrated an important role of these ratios for corrective activity of the investigated mixtures of polar lipids.
3.1. Pharmacological Studies on the Model of Alloxan Diabetes
Table 3 shows that induction of alloxan diabetes increased blood glucose in negative control animals by 3.4